基于铁(III)大环配合物的锌(II)响应型磁共振成像探针
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
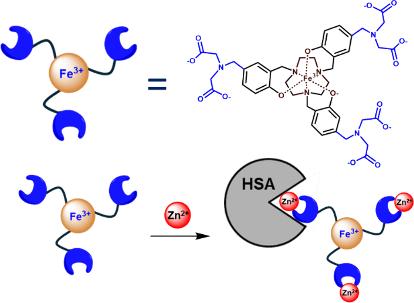
开发反应性MRI造影剂来检测Zn(II)的波动是一个日益增长的研究领域。在这里,我们描述了一个高自旋Fe(III)配位配合物Fe(ADAPT),作为Fe(III) MRI探针对Zn(II)响应的第一个例子之一。六坐标铁(ADAPT)包含一个苯酚附加的1,4,7-三氮杂环壬烷(TACN)配体框架,苯酚基团与Zn(II)结合片段相连。Fe(ADAPT)缺乏可交换的内球水,因此依赖于第二球和外球水的相互作用来实现质子弛豫。在pH 7.4和37°C的生理条件下,Fe(ADAPT)在72小时内对过量的Zn(II)具有高度的动力学惰性。在200 μM Fe(III)探针存在2等量Zn(II)的情况下,观察到弛豫率增加了约80%。铁(ADAPT)、锌(II)和人血清白蛋白之间的三元配合物导致弛豫度增加近200%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Zn(II)-Responsive MRI Probe Based on an Fe(III) Macrocyclic Complex
The development of responsive MRI contrast agents to detect fluctuations in Zn(II) is a growing area of research. Here we describe a high-spin Fe(III) coordination complex, Fe(ADAPT), as one of the first examples of an Fe(III) MRI probe that is responsive to Zn(II). The six-coordinate Fe(ADAPT) contains a phenolate-appended 1,4,7-triazacyclononane (TACN) ligand framework, with the phenolate groups linked to a Zn(II) binding moiety. Fe(ADAPT) lacks an exchangeable inner-sphere water and thus relies on second- and outer-sphere water interactions for proton relaxation. Fe(ADAPT) is highly kinetically inert under physiological conditions at pH 7.4 and 37 °C and to excess Zn(II) over 72 h. In the presence of 2 equiv of Zn(II) with a 200 μM Fe(III) probe, an increase in relaxivity of ∼80% is observed. A ternary complex among Fe(ADAPT), Zn(II), and human serum albumin leads to a nearly 200% increase in relaxivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


