CuFeO2/Fe2O3异质结构光催化- fenton协同反应降解盐酸四环素
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
非均相光催化- fenton协同反应因其对太阳能的高效利用和较高的H2O2活化活性而被认为是一种很有前途的有机污染物降解技术。采用水热法制备了具有Fe3+/Fe2+和Cu2+/Cu+双氧化还原偶的CuFeO2/Fe2O3异质结构。与原始的CuFeO2和Fe2O3相比,CuFeO2/Fe2O3异质结构的光催化- fenton协同反应活性大大提高,在135 min内对盐酸四环素(TCH)的去除率为97.04 %。同时,CuFeO2/Fe2O3异质结构体系在2-9的广泛pH范围内表现出优异的稳定性和降解TCH的效率。TCH的高效降解活性主要归功于其内置氧化还原对(Fe3+/Fe2+和Cu2+/Cu+)的特性,有效地促进了H2O2的活化,有利于形成更多活性物质。此外,理论计算表明,在CuFeO2和Fe2O3的异质界面处建立了强大的内置电场,促进了光致电子和空穴的有效分离和转移。这反过来又促进了Cu2+的原位再循环为Cu+和Fe3+的原位再循环为Fe2+。本研究为制备新型异质结构光催化fenton修复含抗生素污染物废水提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
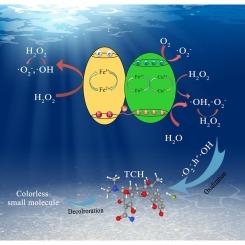
Photocatalytic-Fenton synergistic reaction of CuFeO2/Fe2O3 heterostructure for tetracycline hydrochloride degradation
The heterogeneous photocatalytic-Fenton synergistic reaction is regarded as a promising technique for organic pollutants degradation due to its efficient utilization of solar energy and high H2O2 activation activity. Herein, a CuFeO2/Fe2O3 heterostructure with Fe3+/Fe2+ and Cu2+/Cu+ double redox couples were fabricated via a hydrothermal process. Compared with the pristine CuFeO2 and Fe2O3, the CuFeO2/Fe2O3 heterostructure displays much-increased photocatalytic-Fenton synergistic reaction activity, with tetracycline hydrochloride (TCH) removal by 97.04 % within 135 min. Meanwhile, the CuFeO2/Fe2O3 heterostructure system exhibits excellent stability and efficiency in degrading TCH across a wide pH range of 2–9. The efficient TCH degradation activity should be mainly ascribed to the characteristics of the built-in redox couples (Fe3+/Fe2+ and Cu2+/Cu+) effectively promote the activation of H2O2, which is highly benefits the formation of more active species. Additionally, theoretical calculations elucidate the establishment of strong built-in electric field at the heterointerface of CuFeO2 and Fe2O3, encouraging the effective separation and transfer of photoinduced electrons and holes. This, in turn, promotes the in-situ recycling of Cu2+ to Cu+ and Fe3+ to Fe2+. This work provides new insights for fabricating novel heterostructure for the effective photocatalytic-Fenton remediation of wastewater containing antibiotic contaminants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


