炔叠氮化官能团促进的五元环和七元环的构建:喹啉融合三唑-氮卓类化合物的获取。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
采用分子内1,3-偶极叠氮-炔环加成策略合成了新型喹啉-三唑-氮平衍生物。该方法在不需要金属催化剂或添加剂的情况下,以高收率(65-87%)合成五元环和七元环具有相当大的潜力。此外,该方法适用于吡啶和四酮基加合物,以获得三唑-氮卓衍生物。这种有趣的化学提供了许多优点,包括无金属和无添加剂的工艺,适用于克级合成,高原子经济性和容易获得高产量的潜在药理化合物的各种文库。本文章由计算机程序翻译,如有差异,请以英文原文为准。
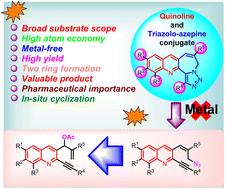
Construction of five- and seven-membered rings facilitated by alkyne–azide functionality: access to quinoline fused triazolo-azepines†
The synthesis of novel quinoline-fused triazolo-azepine derivatives has been reported using an intramolecular 1,3-dipolar azide–alkyne cycloaddition strategy. This method possesses considerable potential to synthesize five- and seven-membered rings in high yields (65–87%) without the necessity of metal catalysts or additives. Additionally, this methodology was applicable to pyridine and tetralone based adducts to afford triazolo-azepine derivatives. This intriguing chemistry offers numerous advantages, including metal-free and additive-free processes, applicability to gram scale synthesis, high atom economy and easy access to a diverse library of potential pharmacological compounds in high yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


