一种常见的遗传人类PCSK9种系变异通过LRP1受体驱动乳腺癌转移
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
识别有转移性复发风险的患者是一项关键的医疗需求。我们在多种乳腺癌患者队列中发现了一种常见的蛋白转化酶subtilisin/kexin type 9 (PCSK9) (rs562556, V474I)的错意种系变异,该变异与生存率降低有关。这种功能获得的单核苷酸变异在小鼠中的遗传模型显示,它可以促进乳腺癌转移。相反,在多种乳腺癌模型中,宿主PCSK9缺失减少了转移性定植。宿主PCSK9通过靶向肿瘤低密度脂蛋白受体相关蛋白1 (LRP1)受体,抑制促进转移的基因XAF1和USP18,促进肺转移起始事件,增强转移增殖能力。抗体介导的PCSK9治疗性抑制在多种模型中抑制乳腺癌转移。在一项大型瑞典早期乳腺癌队列研究中,rs562556纯合子在15年时远处转移性复发的风险为22%,而非纯合子的风险为2%。我们的研究结果表明,一种常见的遗传基因改变控制着乳腺癌的转移,并预测了乳腺癌的生存——揭示了乳腺癌转移的遗传基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
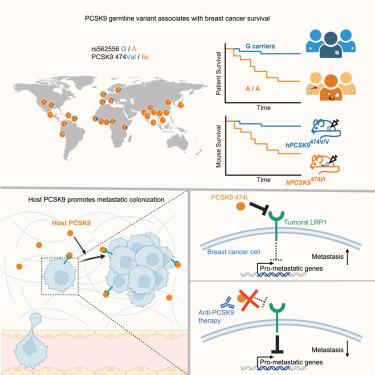
A commonly inherited human PCSK9 germline variant drives breast cancer metastasis via LRP1 receptor
Identifying patients at risk for metastatic relapse is a critical medical need. We identified a common missense germline variant in proprotein convertase subtilisin/kexin type 9 (PCSK9) (rs562556, V474I) that is associated with reduced survival in multiple breast cancer patient cohorts. Genetic modeling of this gain-of-function single-nucleotide variant in mice revealed that it causally promotes breast cancer metastasis. Conversely, host PCSK9 deletion reduced metastatic colonization in multiple breast cancer models. Host PCSK9 promoted metastatic initiation events in lung and enhanced metastatic proliferative competence by targeting tumoral low-density lipoprotein receptor related protein 1 (LRP1) receptors, which repressed metastasis-promoting genes XAF1 and USP18. Antibody-mediated therapeutic inhibition of PCSK9 suppressed breast cancer metastasis in multiple models. In a large Swedish early-stage breast cancer cohort, rs562556 homozygotes had a 22% risk of distant metastatic relapse at 15 years, whereas non-homozygotes had a 2% risk. Our findings reveal that a commonly inherited genetic alteration governs breast cancer metastasis and predicts survival—uncovering a hereditary basis underlying breast cancer metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


