通过邻近诱导方式调节磷酸化用于癌症治疗
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
蛋白质的异常磷酸化可导致各种疾病,特别是癌症。因此,开发用于精确调节蛋白质磷酸化的小分子具有很大的药物设计潜力。虽然传统的激酶/磷酸酶小分子调节剂已经取得了一些成功,但实现精确的磷酸化调节已被证明是具有挑战性的。异双功能分子的出现,如磷酸化诱导嵌合小分子(phics)和磷酸酶募集嵌合体(PHORCs),具有邻近诱导模式,有望通过特异性募集激酶或磷酸酶到感兴趣的蛋白质上,带来突破。在此,我们总结了在癌症中异常磷酸化的药物靶点,并强调了在癌症治疗中纠正磷酸化的潜力。通过报道的靶向磷酸化调控的异双功能分子的案例,我们强调了这些分子目前的设计策略和特点。我们还系统阐述了异常磷酸化靶点与癌症之间的联系,以及开发用于磷酸化调控的异双功能分子药物的现有挑战和未来研究方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
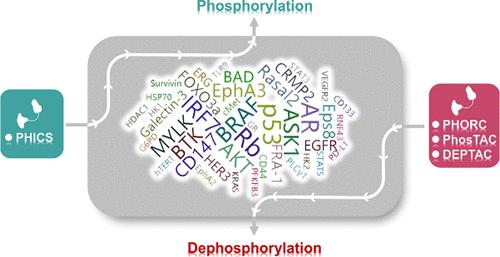
Modulating Phosphorylation by Proximity-Inducing Modalities for Cancer Therapy
Abnormal phosphorylation of proteins can lead to various diseases, particularly cancer. Therefore, the development of small molecules for precise regulation of protein phosphorylation holds great potential for drug design. While the traditional kinase/phosphatase small-molecule modulators have shown some success, achieving precise phosphorylation regulation has proven to be challenging. The emergence of heterobifunctional molecules, such as phosphorylation-inducing chimeric small molecules (PHICSs) and phosphatase recruiting chimeras (PHORCs), with proximity-inducing modalities is expected to lead to a breakthrough by specifically recruiting kinase or phosphatase to the protein of interest. Herein, we summarize the drug targets with aberrant phosphorylation in cancer and underscore the potential of correcting phosphorylation in cancer therapy. Through reported cases of heterobifunctional molecules targeting phosphorylation regulation, we highlight the current design strategies and features of these molecules. We also provide a systematic elaboration of the link between aberrantly phosphorylated targets and cancer as well as the existing challenges and future research directions for developing heterobifunctional molecular drugs for phosphorylation regulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


