患者来源的胶质母细胞瘤类器官作为评估临床CAR-T细胞治疗反应的实时化身
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
患者来源的肿瘤类器官已被用于疾病建模和临床前研究,但很少应用于临床中实时帮助解释患者治疗反应。我们最近在一项首次在人体内进行的i期研究中证明了双靶向嵌合抗原受体(CAR -T)细胞(EGFR-IL13Rα2 CAR-T细胞)治疗复发性胶质母细胞瘤患者的早期疗效信号。在我们的1期研究中,我们分析了6组患者源性胶质母细胞瘤类器官(GBOs)与相同的自体CAR-T细胞产品同时治疗。我们发现CAR-T细胞治疗导致GBOs中肿瘤细胞的靶抗原减少和细胞溶解,其程度与患者脑脊液(CSF)中检测到的CAR-T细胞植入相关。此外,随着时间的推移,gbo中的细胞因子释放模式反映了患者CSF样本中的细胞因子释放模式。我们的研究结果突出了独特的试验设计和GBOs作为实时评估CAR-T细胞生物活性和了解免疫治疗疗效的宝贵平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
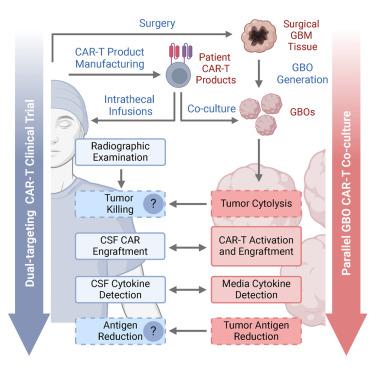
Patient-derived glioblastoma organoids as real-time avatars for assessing responses to clinical CAR-T cell therapy
Patient-derived tumor organoids have been leveraged for disease modeling and preclinical studies but rarely applied in real time to aid with interpretation of patient treatment responses in clinics. We recently demonstrated early efficacy signals in a first-in-human, phase 1 study of dual-targeting chimeric antigen receptor (CAR)-T cells (EGFR-IL13Rα2 CAR-T cells) in patients with recurrent glioblastoma. Here, we analyzed six sets of patient-derived glioblastoma organoids (GBOs) treated concurrently with the same autologous CAR-T cell products as patients in our phase 1 study. We found that CAR-T cell treatment led to target antigen reduction and cytolysis of tumor cells in GBOs, the degree of which correlated with CAR-T cell engraftment detected in patients’ cerebrospinal fluid (CSF). Furthermore, cytokine release patterns in GBOs mirrored those in patient CSF samples over time. Our findings highlight a unique trial design and GBOs as a valuable platform for real-time assessment of CAR-T cell bioactivity and insights into immunotherapy efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


