从人多能干细胞生成自组织神经肌肉骨骼三组织类器官
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
人体机能需要不同组织之间的相互作用。一个重要的串扰是在神经肌肉骨骼(NMS)轴涉及神经、肌肉和骨骼组织,这是具有挑战性的模型使用人类细胞。在这里,我们描述了通过共同发育策略从人类多能干细胞生成三维NMS三组织类器官(hNMSOs)。染色、单核RNA测序和空间转录组分析揭示了神经、肌肉和骨骼谱系在单个类器官内的共同出现和自组织,hnmso的神经结构域获得了腹侧特异性的身份,并产生了支配骨骼肌的运动神经元。hnmso的神经、肌肉和骨骼区域在发育过程中成熟并建立了功能连接。值得注意的是,结构、功能和转录组学分析显示,hnmso中的骨骼支撑有利于人类肌肉的发育。hnmso模型也揭示了病理性骨骼变性后的神经肌肉改变。总之,我们的研究为未来人类NMS串扰和异常的研究提供了一个可访问的实验模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
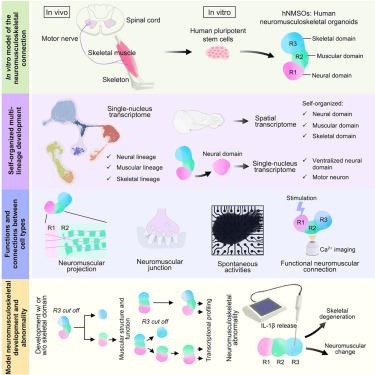
Generation of self-organized neuromusculoskeletal tri-tissue organoids from human pluripotent stem cells
The human body function requires crosstalk between different tissues. An essential crosstalk is in the neuromusculoskeletal (NMS) axis involving neural, muscular, and skeletal tissues, which is challenging to model using human cells. Here, we describe the generation of three-dimensional, NMS tri-tissue organoids (hNMSOs) from human pluripotent stem cells through a co-development strategy. Staining, single-nucleus RNA sequencing, and spatial transcriptome profiling revealed the co-emergence and self-organization of neural, muscular, and skeletal lineages within individual organoids, and the neural domains of hNMSOs obtained a ventral-specific identity and produced motor neurons innervating skeletal muscles. The neural, muscular, and skeletal regions of hNMSOs exhibited maturation and established functional connections during development. Notably, structural, functional, and transcriptomic analyses revealed that skeletal support in hNMSOs benefited human muscular development. Modeling with hNMSOs also unveiled the neuromuscular alterations following pathological skeletal degeneration. Together, our study provides an accessible experimental model for future studies of human NMS crosstalk and abnormality.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


