在拟南芥中,SUMOylation控制肽加工以产生与损伤相关的分子模式
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
在受到损伤时,哺乳动物和植物细胞都通过感知内源性损伤相关分子模式(DAMPs)激活一种生存机制。植物激发肽(Peps)是一种具有代表性的DAMP,从它们的前体(PROPEPs;Pep的前体是通过metacaspase (MCs)的裂解而产生的,但Pep的控制机制尚不清楚。在这里,我们发现拟南芥中的几种PROPEP1是summoylation的底物,Ca2+在SUMO E3连接酶SAP和MIZ1结构域连接酶(SIZ1)的促进下上调PROPEP1的summoylation。在PROPEP1上的sumo化位点或其蛋白酶MC4上的sumo相互作用基序(SIMs)发生突变,减少了PROPEP1-MC4的结合和PROPEP1的切割。过度表达野生型的PROPEP1,而不是summoylation缺陷型的PROPEP1,增强了植物对细胞壁损伤的耐受性。一致地,SIZ1参与PROPEP1加工和细胞壁损伤反应。这些发现支持了SUMOylation通过MC4促进PROPEP1切割的观点,并为真核细胞中如何控制DAMP的产生提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
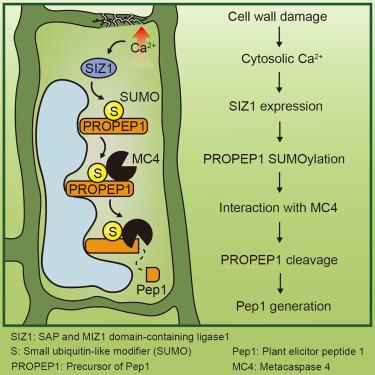
SUMOylation controls peptide processing to generate damage-associated molecular patterns in Arabidopsis
Upon injury, both mammalian and plant cells activate a survival mechanism by sensing endogenous damage-associated molecular patterns (DAMPs). Plant elicitor peptides (Peps), a representative DAMP, are released from their precursors (PROPEPs; Precursors of Peps) through cleavage by metacaspases (MCs), but the control of Pep generation remains unclear. Here, we discovered that several PROPEPs in Arabidopsis thaliana are substrates for SUMOylation and that Ca2+ upregulates PROPEP1 SUMOylation, facilitated by the SUMO E3 ligase SAP and MIZ1 domain-containing ligase1 (SIZ1). Mutations at the SUMOylation site on PROPEP1, or at the SUMO-interacting motifs (SIMs) on its protease MC4, reduced the PROPEP1-MC4 association and PROPEP1 cleavage. Overexpression of the wild-type form, but not the SUMOylation-defective variant of PROPEP1, enhanced plant tolerance to cell wall damage. Consistently, SIZ1 contributes to PROPEP1 processing and cell wall damage responses. These findings support the idea that SUMOylation promotes PROPEP1 cleavage via MC4 and provide insights into how DAMP generation is controlled in eukaryotic cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


