基于区域选择性原子层沉积的双电极平台光催化剂反应选择性空间控制研究
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
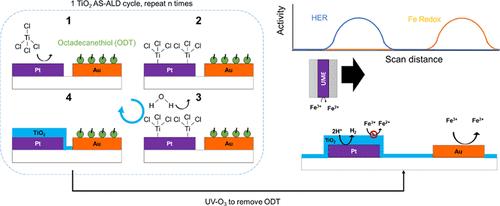
光催化水裂解是一种很有前途的低成本、绿色氢气生产途径。然而,这种方法目前在太阳能到氢的转换效率方面受到限制。效率损失的一个主要来源是由于不期望的副反应和反反应的高速率,这是加剧邻近的氧化和还原位点。纳米氧化物涂层以前被用来选择性地阻止不需要的反应物到达活性位点;然而,包裹整个光催化剂颗粒的涂层限制了活性,因为它不能促进两个半反应。在本研究中,采用区域选择性原子层沉积(AS-ALD)技术将半透性TiO2膜选择性沉积在模型金属助催化剂上,以提高反应选择性,同时保持较高的整体活性。Pt和Au分别被用作还原和氧化共催化剂位点,其中Au通过自组装的硫醇单层使ALD生长失活,而TiO2被涂覆在Pt位点上。单金属薄膜电极的电分析测量表明,tio2封装的Pt有效地抑制了不需要的H2氧化和Fe(II)/Fe(III)氧化还原反应,同时仍然允许所需的析氢反应(HER)。利用扫描电化学显微镜(SECM)进一步评估了包含Au和Pt微电极图案交错阵列的平面模型光催化剂平台,证明了AS-ALD成功地在双反应现场(光)电催化系统中实现了局部反应选择性。最后,使用具有独立电位控制的交叉指状微电极表明,与未涂覆的对照样品相比,选择性沉积的TiO2涂层可以将邻近活性位点的反反应速率抑制一个数量级。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Toward Spatial Control of Reaction Selectivity on Photocatalysts Using Area-Selective Atomic Layer Deposition on the Model Dual Site Electrocatalyst Platform
Photocatalytic water splitting is a promising route to low-cost, green H2. However, this approach is currently limited in its solar-to-hydrogen conversion efficiency. One major source of efficiency loss is attributed to the high rates of undesired side and back reactions, which are exacerbated by the proximity of neighboring oxidation and reduction sites. Nanoscopic oxide coatings have previously been used to selectively block undesired reactants from reaching active sites; however, a coating encapsulating the entire photocatalyst particle limits activity as it cannot facilitate both half-reactions. In this work, area selective atomic layer deposition (AS-ALD) was used to selectively deposit semipermeable TiO2 films onto model metallic cocatalysts for enhancing reaction selectivity while maintaining a high overall activity. Pt and Au were used as exemplary reduction and oxidation cocatalyst sites, respectively, where Au was deactivated toward ALD growth through self-assembled thiol monolayers while TiO2 was coated onto Pt sites. Electroanalytical measurements of monometallic thin film electrodes showed that the TiO2-encapsulated Pt effectively suppressed undesired H2 oxidation and Fe(II)/Fe(III) redox reactions while still permitting the desired hydrogen evolution reaction (HER). A planar model photocatalyst platform containing patterned interdigitated arrays of Au and Pt microelectrodes was further assessed using scanning electrochemical microscopy (SECM), demonstrating the successful use of AS-ALD to enable local reaction selectivity in a dual-reaction-site (photo)electrocatalytic system. Finally, interdigitated microelectrodes having independent potential control were used to show that selectively deposited TiO2 coatings can suppress the rate of back reactions on neighboring active sites by an order of magnitude compared with uncoated control samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


