了解硫酸盐转运现象在电化学酸回收废MgSO4:建模方法
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
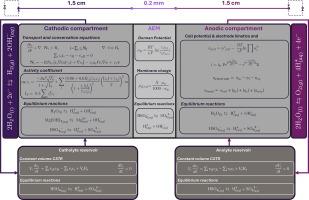
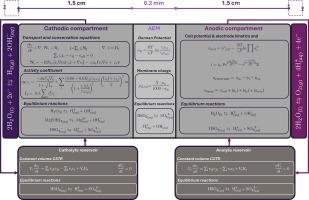
电化学膜分离是一种有效的技术,可以最大限度地减少工艺废物,特别是当特定的废物缓解做法不能提供资源回收途径时。初级镍生产产生相当数量的硫酸镁废液,这些废液通常储存在蒸发池中或排入水体。通过水裂解对这些硫酸镁溶液进行电化学处理,可以产生硫酸和氢氧化镁,并在镍生产过程中重复使用,减少了对额外工艺试剂的需求。这种水分解技术涉及电化学电池内多离子的传输。由于硫酸盐在整个电解槽中的形态变化,研究废液浓度下的硫酸盐运输带来了挑战,这可能会显著影响法拉第效率。在这项工作中,建立了一个基于有限元法的一维数值模型,以了解硫酸镁水电解过程中所有离子的通量行为。结果表明,质子反扩散,而不是迁移,负责85%的总质子泄漏 %。结果还表明,在评价条件下,HSO4−是平衡膜电荷的主要物质。高电流密度增加了硫酸盐膜形成的速率,而当MgSO4阴极电解质浓度超过1 M时,对硫酸盐通量的影响最小。模型计算的电解液电位降范围为0.83 ~ 1.5 V。模型结果与我们之前发表的实验结果进行了验证,并证明了与实验酸采收率的良好一致性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Understanding sulfate transport phenomena during electrochemical acid recovery from waste MgSO4: A modelling approach
Electrochemical membrane separation is an effective technique for minimizing process waste, particularly when waste-specific mitigation practices do not offer a pathway for resource recovery. Primary nickel production generates considerable amounts of magnesium sulfate waste solutions, which are typically stored in evaporation ponds or disposed into water bodies. By electrochemically treating these magnesium sulfate solutions through water splitting, sulfuric acid and magnesium hydroxide can be produced and reused within the nickel production process, reducing the need for additional process reagents. This water splitting technology involves the transport of multi-ionic species within the electrochemical cell. Investigating sulfate transport at waste solution concentrations poses challenges due to its varying speciation throughout the electrolyser, which can significantly influence faradaic efficiency. In this work, a 1-D numerical model based on a Finite Element Method is developed to understand the flux behaviour of all of ions involved during magnesium sulfate water electrolysis. Results showed that proton back-diffusion, rather than migration, is responsible for 85 % of the total proton leakage. It also revealed that HSO4− is the major species balancing the membrane charge under the evaluated conditions. High current densities increased the rate of sulfate membrane speciation, while increasing the MgSO4 catholyte concentration beyond 1 M showed minimal impact on sulfate flux. The electrolyte potential drop calculated by the model ranged from 0.83 to 1.5 V. The model results are validated against our previously published experimental work and demonstrated a good agreement with experimental acid recovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


