一种结合磁分选和有创分离的微流控芯片,用于循环肿瘤细胞的分离、培养和端粒酶分析。
IF 6.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
循环肿瘤细胞(CTCs)是肿瘤转移的重要指标,对早期诊断、疾病监测和治疗反应评估至关重要。然而,它们极低的浓度和分离技术的复杂性对捕获和分析ctc构成了重大挑战。在这项研究中,我们开发了一种新型的微流控系统,将磁捕获和侵入性筛选集成在单个微流控芯片上。通过将带正电荷的磁性纳米颗粒附着在带负电荷的ctc上,芯片内ctc的磁性分离有效地消除了血细胞的干扰。总共2ml的血液样本可以在3分钟内处理,达到84%的令人印象深刻的肿瘤捕获效率。利用该芯片,我们还成功实现了CTCs的长期培养,并在11例结直肠癌患者的血液样本中鉴定出具有高活性和侵袭潜力的CTCs。最后,我们在微流控芯片上分析了培养ctc的端粒酶活性。恶性肿瘤组ctc的侵袭电位和端粒酶活性明显高于良性肿瘤组(P本文章由计算机程序翻译,如有差异,请以英文原文为准。
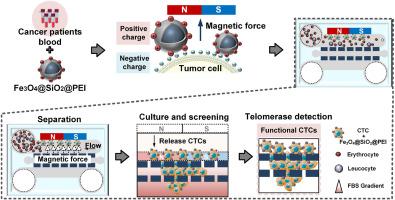
A microfluidic chip incorporating magnetic sorting and invasive separation for isolation, culture and telomerase analysis of circulating tumor cells
Circulating tumor cells (CTCs) are a crucial indicator of cancer metastasis, and are vital for early diagnosis, disease monitoring, and treatment response evaluation. However, their extremely low concentration and the complexities of isolation techniques pose a significant challenge in capturing and analyzing CTCs. In this study, we developed a novel microfluidic system that integrates magnetic capture and invasive screening onto a single microfluidic chip. By attaching positively charged magnetic nanoparticles to negatively charged CTCs, the magnetic separation of CTCs within the chip effectively eliminates interference from blood cells. A total of 2 mL blood sample can be processed within 3 min, achieving an impressive tumor capture efficiency of 84 %. Using the chip, we also successfully achieved long-term culture of CTCs, and identified CTCs with high activity and invasive potential in blood samples from 11 patients with colorectal cancer. Finally, we analyzed telomerase activity in cultured CTCs on the microfluidic chip. Significantly higher invasive potential and telomerase activity were observed in CTCs from the malignant tumor group compared to the benign group (P < 0.01), highlighting their increased aggressiveness. This study offers a novel approach for efficient CTCs isolation, culture, and telomerase analysis, clarifying the crucial role of telomerase in tumor metastasis and providing profound insights for future research on telomerase-targeted tumor metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Talanta
化学-分析化学
CiteScore
12.30
自引率
4.90%
发文量
861
审稿时长
29 days
期刊介绍:
Talanta provides a forum for the publication of original research papers, short communications, and critical reviews in all branches of pure and applied analytical chemistry. Papers are evaluated based on established guidelines, including the fundamental nature of the study, scientific novelty, substantial improvement or advantage over existing technology or methods, and demonstrated analytical applicability. Original research papers on fundamental studies, and on novel sensor and instrumentation developments, are encouraged. Novel or improved applications in areas such as clinical and biological chemistry, environmental analysis, geochemistry, materials science and engineering, and analytical platforms for omics development are welcome.
Analytical performance of methods should be determined, including interference and matrix effects, and methods should be validated by comparison with a standard method, or analysis of a certified reference material. Simple spiking recoveries may not be sufficient. The developed method should especially comprise information on selectivity, sensitivity, detection limits, accuracy, and reliability. However, applying official validation or robustness studies to a routine method or technique does not necessarily constitute novelty. Proper statistical treatment of the data should be provided. Relevant literature should be cited, including related publications by the authors, and authors should discuss how their proposed methodology compares with previously reported methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


