立体定向和立体发散的烯丙基-烯丙基偶联:邻叔碳和全碳四元立体中心的构建
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们证明了在支链碳酸盐烯丙基酯和α-硅基-γ、γ-二烷基烯丙基硼酯之间铱催化的高度立体定向和立体发散的烯丙基-烯丙基偶联。用3,5-(CF3)2-苯基锂作为α-硅基-γ,γ-二烷基烯基硼酯硼基团的活化剂,得到了对映体富集(E)-1-硅基取代的1,5-二烯,其叔立体中心与全碳四元中心相邻。值得注意的是,这种方法可以通过排列所采用的α-硅基-γ、γ-二烷基烯丙基硼酯和配体的手性来获得所有四种可能的立体异构体。密度泛函理论计算为观察到的选择性提供了机理上的见解。过渡态能量的评估为实验结果提供了理论支持,并确定了控制化学选择性、非映体选择性和对映体选择性的关键因素。此外,我们通过将所得到的富集对映体(E)-1-硅基取代的1,5-二烯转化为各种富集对映体的化合物,展示了该方法的多功能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
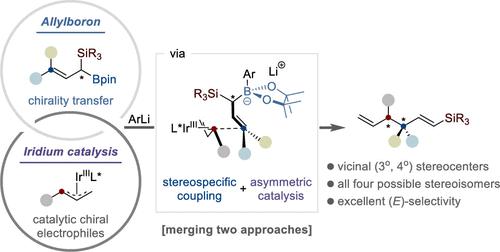
Stereospecific and Stereodivergent Allyl–Allyl Coupling: Construction of Vicinal Tertiary and All-Carbon Quaternary Stereocenters
We demonstrate an iridium-catalyzed highly stereospecific and stereodivergent allyl–allyl coupling between branched allyl carbonates and α-silyl-γ,γ-dialkyl allylboronic esters. When 3,5-(CF3)2-phenyllithium was used as an activator of the boron group of α-silyl-γ,γ-dialkyl allylboronic esters, the enantioenriched (E)-1-silyl-substituted 1,5-dienes bearing a tertiary stereocenter adjacent to the all-carbon quaternary center were obtained. Notably, this approach allows access to all four possible stereoisomers by permuting the chirality of the employed α-silyl-γ,γ-dialkyl allylboronic esters and ligands. Density functional theory calculations provide mechanistic insights into the observed selectivities. Evaluation of transition state energies offers theoretical support for the experimental outcomes and identifies the key factors controlling the chemo-, diastereo-, and enantioselectivity. Furthermore, we demonstrate synthetic applications by transforming the resulting enantioenriched (E)-1-silyl-substituted 1,5-dienes into a variety of enantioenriched compounds, showcasing the versatility of this approach.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


