氧化负载钯双功能热催化CO2加氢的机理
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
将二氧化碳加氢成清洁燃料和碳氢化合物,如甲烷、甲酸和C2+产品,是减少人为二氧化碳排放的可行策略。基于密度泛函理论计算,我们阐明了MgO或CaO负载的Pd纳米颗粒加氢CO2的机理。为了对活性和选择性CO2加氢催化剂的合理设计提供基本的见解,我们将Pd(氢活化剂)和MgO或CaO (CO2粘合剂)结合起来。我们发现Pd优先结合和解离H2,并且Pd-氧化物界面激活CO2,完成双功能CO2加氢反应。与Pd-MgO界面相比,Pd-CaO界面能强结合CO2。因此,减弱的C-O键使CO2的氧加氢,激活CO2加氢的羧基途径,生成CO和甲醇。相比之下,由于与CO2的相互作用相对较弱,在Pd-MgO催化剂中通过CO2碳的直接加氢形成甲酸途径。Pd/MgO在热力学上更容易产生甲酸和甲醇。我们的研究结果表明,催化剂-CO2相互作用控制了金属/氧化物类非均相催化剂热催化CO2加氢的整个反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
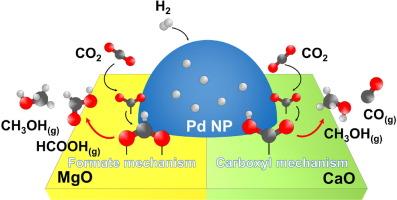
Mechanistic insight into bifunctional thermocatalytic CO2 hydrogenation by Oxide-Supported Palladium
Hydrogenating CO2 into clean fuels and hydrocarbons such as methane, formic acid, and C2+ products is a viable strategy for mitigating anthropogenic carbon dioxide. Based on density functional theory calculations, we elucidate the mechanism of CO2 hydrogenation by Pd nanoparticles supported on MgO or CaO. To provide fundamental insight into the rational design of active and selective CO2 hydrogenation catalysts, we combined Pd, a hydrogen activator, and MgO or CaO, a CO2 binder. We found that Pd preferentially binds and dissociates H2, and the Pd-oxide interface activates CO2, completing a bifunctional CO2 hydrogenation reaction. The Pd-CaO interface strongly binds CO2 compared to the Pd-MgO interface. Therefore, the weakened C-O bond enables hydrogenation of the oxygen of CO2, activating the carboxyl pathway of CO2 hydrogenation and producing CO and methanol. In contrast, the formate pathway through direct hydrogenation of the carbon of CO2 operates in the Pd-MgO catalyst due to the relatively weak interaction with CO2. Producing formic acid and methanol is thermodynamically more accessible in Pd/MgO. Our results show that the catalyst-CO2 interaction steers the overall reaction pathway of thermocatalytic CO2 hydrogenation by metal/oxide class heterogeneous catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


