家族性地中海热缺铁:来自JIR队列的211名成年患者的研究
IF 10.1
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
家族性地中海热(FMF)是世界上最常见的与MEFV突变相关的单基因自身炎症性疾病。患者表现为反复发作和自限性发热,腹部和胸部疼痛[1]。除了慢性炎症患者可能有炎性小细胞性贫血[2]外,贫血与FMF之间没有特异性关联。然而,慢性贫血可导致疲劳,而疲劳是FMF发作的触发因素[2,5]。因此,贫血引起的疲劳患者可能有更多的疾病发作和更差的生活质量[10]。即使没有贫血,缺铁也会引起疲劳。疲劳也常见于FMF bb0。因此,寻找没有贫血的缺铁可能是有益的,因为它是FMF中可治愈的疲劳原因之一,特别是疲劳可能被认为是其发作的触发因素。尽管对铁状态的各种实验室评估是可用的,血清铁蛋白(一种与组织铁蛋白平衡的循环蛋白)是检测孤立铁缺乏症最敏感和最特异的测试。然而,在这种自身炎症性疾病的急性炎症发作中,血清铁蛋白的评估可能会受到炎症背景的影响,因为在正常或高比率的情况下,它可能无法告知真正缺乏铁。我们的目的是评估FMF中缺铁的患病率及其与临床特征、实验室参数和治疗的关系。2016年至2023年,对法国国家FMF参考中心前瞻性随访的FMF患者进行回顾性评估,分析铁缺乏症的患病率及其与临床特征、实验室参数和治疗方法的关系。根据我院实验室铁蛋白阈值(铁蛋白27.0 ng/mL)确定缺铁,采集测量均匀。从随访的医疗记录中收集铁蛋白水平。考虑到本研究的回顾性,我们收集了患者临床图表中报告的内容;然而,一些相关参数(即转铁蛋白饱和度,血清转铁蛋白受体水平)未包括在分析中,因为它们不在常规护理中进行。所有FMF均显示两个致病外显子10 MEFV突变M694V (pMet694Val)和/或M694I (pMet694Ile)。在收集知情同意书后,我们从青少年炎症性风湿病(JIR)队列中提取数据,这是一个由国家信息与自由委员会(CNIL,授权号914677)授权的国际多中心数据库。统计学方面,探索性比较临床特征,根据有无缺铁(铁蛋白27.0 ng/mL),酌情采用连续或分类变量t检验。之后,建立多变量回归模型,探讨铁蛋白含量为27.0 ng/mL患者的临床风险特征。由单变量分析开始的变量选择过程;任何具有显著单变量检验的变量都被检验为多变量分析的可能候选者。此外,根据其临床相关性对变量进行多变量分析。在这个多步骤过程的最后,我们建立了多变量模型,提供了铁蛋白含量为27.0 ng/mL的患者临床风险概况的OR估计。p值<; 0.05认为有统计学意义。数据缺失的患者被排除在分析之外。统计软件包社会科学(SPSS 17.0版本,SPSS Inc.)用于所有分析。共纳入211例FMF,多数为女性(60.2%),平均年龄41.34岁[18-89]。在我们数据库中报告的208例铁蛋白值患者中,67例(31.80%)血清铁蛋白水平为27 ng/mL,被定义为缺铁。其中女性61例(91.04%),平均年龄36.81岁[18-89]。67例缺铁患者中,42例(62.69%)表现为贫血(Hb < 13.0 g/dL),其中女性40例,男性2例。缺铁患者Hb (p < 0.001)、平均红细胞体积(MCV) (p = 0.002)、BMI (p = 0.044)、体重(p < 0.001)、秋水仙碱日剂量(p = 0.015)、肌酐(p = 0.005)均低于无缺铁患者(p = 0.011)。两组在炎症标志物方面没有差异。特别是,52.24%的含铁蛋白27的FMF炎症标志物正常(表S1)。82例男性中6例血清铁蛋白水平为27 ng/mL。与不缺铁的患者相比,这些患者在主要特征上没有显著差异(表S2)。根据缺铁情况分析Hb值和秋水仙碱日剂量的差异(图S1)。 在我们的队列中,我们建立了单因素和多因素回归模型来评估铁蛋白<; 27 ng/mL患者的临床风险概况(表1)。基于单因素分析、临床相关性,同时考虑到铁蛋白<; 27 ng/mL患者的数量,我们建立了两个多因素回归模型。一个模型包括年龄、女性性别、CRP、SAA、BMI和秋水仙碱剂量,而另一个模型包括年龄、女性性别、CRP、SAA、秋水仙碱剂量和生物疾病改善抗风湿药物(bDMARDs)。尽管单因素分析不显著,但考虑到炎症过程对铁蛋白水平的可能影响,我们在模型中加入了CRP和SAA。考虑到更严重的患者可能使用这些药物治疗,我们在模型中添加了bdmard。在第一个模型中,年龄(OR: 0.97, 95% CI: 0.95-1.00, p = 0.041)和女性(OR: 13.77, 95% CI: 4.96-38.18, p < 0.001)与铁蛋白27 ng/mL的存在显著且独立相关。同样,在另一个模型中,年龄(OR: 0.97, 95% CI: 0.95-1.00, p = 0.015)和女性(OR: 16.15, 95% CI: 5.67-46.00, p < 0.001)显著预测铁蛋白27 ng/mL。表1。回归分析评估以铁蛋白27 ng/mL为特征的患者的临床风险概况。临床variablesORSE95% CIpFerritin & lt; 27 ng / mLUnivariate analysisAge0.980.0090.95-1.000.063Female sex11.890.4604.83 - 29.28 & lt; 0.001 hb0.620.0990.51 - 0.75 & lt; 0.001 hb & lt; 13.0 g / dl3.010.3101.64 - 5.53 & lt; 0.001 rbc0.470.2710.28-0.810.006mcv0.890.0360.83-0.960.002crp0.980.0100.96-1.000.088saa0.990.0030.99-1.000.288bmi0.930.0370.87-0.990.045daily秋水仙碱dose1.870.2611.12 - 3.110.016bdmards0.740.3770.36 1.560.431weight0.960.0130.93 - 0.98 & lt; creatinine0.940.0140.91 0.001 - 0.97 - 0.001 & lt;多元analysisAge0.970.0130.95-1.000.041Female sex13.770.5214.96-38.18< 0.001 crp0.990.0140.96 - 1.010.325 saa1.000.0030.99 - 1.000.80 bdmards1.560.3250.83 - 2.960.170多元分析age0.970.0130.95 - 1.000.015 female sex16.150.535.67-46.00< 0.001 crp0.990.0130.96 - 1.010.320saa1.000.0030.99 - 1.000.65每日秋水仙碱剂量1.800.330.94 - 3.470.078 bdmards1.670.520.61 - 4.630.32注:p <; 0.05被认为具有统计学意义。缩写:bDMARDs:生物减病抗风湿药;BMI:身体质量指数;CRP: C反应蛋白;Hb:血红蛋白;M:男性;MCV:平均红细胞体积;N:患者数;SAA:血清淀粉样蛋白A;SD:标准差;W:女性。总的来说,31.8%的FMF显示铁蛋白27 ng/mL,主要是女性和年轻人。铁蛋白可能被认为是铁缺乏检测的主要成分,它已被证明是疲劳的原因,疲劳是FMF发作的一个非常频繁的有利因素。虽然在没有贫血的情况下,缺铁可能是炎症发作的另一个因素,并可能影响患者的生活质量,尽管这方面还不能进行全面的回顾性研究。事实上,为了明确定义FMF患者的“缺铁”状态,也就是在没有贫血的情况下,未来的研究需要评估除铁蛋白外的其他铁代谢参数。根据临床观察和年轻女性的优势,无论其炎症状态如何,我们的第一个假设是,铁蛋白27 ng/mL和相关的铁缺乏,也没有贫血,可能是继发于过度的妇科损失,而不是通过增加富含铁的食物的摄入来完全补偿。由于这项研究是回顾性的,而且没有系统地收集有关患者经期特征的信息,因此无法给出详尽的答案,但当受影响的妇女接受采访时,她们通常报告说,在没有服用避孕药的情况下,经期很长。也有可能是年轻女性少吃动物蛋白。最后,消化道出血,经常使用非甾体抗炎药可能加重,不应忽视。铁缺乏并不是所有患者都导致贫血,但它会导致疲劳,并最终导致FMF发作,这表明需要补充铁。在这里,我们发现含铁蛋白27 ng/mL的FMF患者Hb和BMI较低,并且明显比其他患者年轻,没有升高的炎症生物标志物。有趣的是,他们需要更高剂量的秋水仙碱,主要是每天2毫克。秋水仙碱在减少铁的肠吸收中的作用可能受到质疑。我们的研究并不能回答这个问题,但并不是所有缺铁的病人都服用了最大剂量的秋水仙碱。在我们看来,重要的是纠正FMF缺铁,从治疗铁流失的原因开始,并在可能和耐受的情况下纠正口服摄入。 口服铁与口服秋水仙碱可能会引起消化不适和运输障碍,导致患者停止补充铁。如果口服补充是不可能的,可以提供静脉铁输注。除了铁补充剂,病人还可以得到一份富含铁的食物清单。找出武虚的原因,特别注重妇科出血过多,以减少妇女月经的频率和持续时间。寻找相关的胃疾病也很重要,特别是在可能有过高风险的人群或幽门螺杆菌感染的人群中。我们的研究应被视为“假设生成”,为进一步的具体设计研究提供基础,以更好地阐明这一问题。据我们所知,这将是第一次分析铁蛋白在FMF中的价值。这对于评估虚弱作为炎症性疾病触发的复杂机制可能是重要的。我们强调,单独使用铁蛋白作为铁缺乏的唯一仲裁者必须得到证实,特别是在单基因自身炎症性疾病(如FMF)患者中,如果他们表现出炎症。这项工作强调了测量FMF中铁蛋白水平的重要性,特别是因为该剂量简单且在世界范围内的常规护理中可获得,包括FMF高度流行的一些新兴国家。在存在明显缺铁的情况下,应补充铁来纠正它,并减少任何缺铁迹象,如可能导致FMF发作的虚弱和贫血,并在FMF患者中形成虚弱圈。本文章由计算机程序翻译,如有差异,请以英文原文为准。

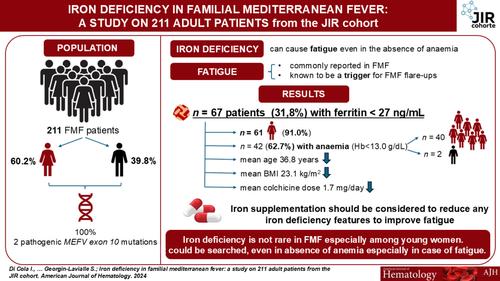
Iron Deficiency in Familial Mediterranean Fever: A Study on 211 Adult Patients From the JIR Cohort
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
15.70
自引率
3.90%
发文量
363
审稿时长
3-6 weeks
期刊介绍:
The American Journal of Hematology offers extensive coverage of experimental and clinical aspects of blood diseases in humans and animal models. The journal publishes original contributions in both non-malignant and malignant hematological diseases, encompassing clinical and basic studies in areas such as hemostasis, thrombosis, immunology, blood banking, and stem cell biology. Clinical translational reports highlighting innovative therapeutic approaches for the diagnosis and treatment of hematological diseases are actively encouraged.The American Journal of Hematology features regular original laboratory and clinical research articles, brief research reports, critical reviews, images in hematology, as well as letters and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


