为什么以及如何控制硫代治疗性寡核苷酸的p -手性:与立体化学组成测定相关的分析挑战
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
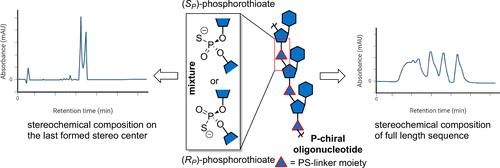
自2016年以来,治疗性寡核苷酸获得批准的数量激增,这类化学模式已成为解决罕见或难治性疾病领域未满足医疗需求的重要工具箱。治疗性寡核苷酸是一种复杂的药物,主要是由于其活性药物成分存在许多寡核苷酸特异性杂质,这在制造过程中具有挑战性。一个不太常见的挑战是控制对手性morpholino和硫代治疗性寡核苷酸的立体化学组成。它们以非立体选择性的方式产生,产生不同立体异构体的混合物,共同形成最终的原料药。因此,立体化学组成的控制在治疗性寡核苷酸的制造过程中具有重要作用,无论是作为过程控制还是最终表征测试。在这方面,我们首先介绍了寡核苷酸治疗的现状,重点是硫代衍生物。然后,我们重点介绍了控制这些药物的立体化学组成的调控框架,简要介绍了立体化学组成对硫代磷酸酯的生物学意义,并讨论了立体化学组成在合成和下游过程中的起源。随后,我们深入分析了用于确定治疗性寡核苷酸立体化学组成的分析方法,包括各种分离模式的液相色谱方法和新兴的现代技术,如离子迁移谱法。此外,还讨论了毛细管电泳、双熔温度和核磁共振波谱在治疗性寡核苷酸的立体化学组成分析中的应用。最后,我们强调了用于将治疗性寡核苷酸切割成更小片段的新型酶促方法,这些片段可以使用标准技术分析立体化学组成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Why and How to Control P-Chirality in Phosphorothioated Therapeutic Oligonucleotides: Analytical Challenges Associated with Determination of Stereochemical Composition
A surge in the approval of therapeutic oligonucleotides since 2016 has made this class of chemical modalities an essential toolbox for addressing unmet medical needs in the realm of rare or difficult-to-treat diseases. Therapeutic oligonucleotides are complex drugs, primarily due to the active pharmaceutical ingredient, which presents numerous oligonucleotide-specific impurities that are challenging to address during the manufacturing process. A less common challenge is the control of the stereochemical composition of P-chiral morpholino and phosphorothioate therapeutic oligonucleotides. These are produced in a non-stereoselective manner, resulting in a mixture of different stereoisomers that collectively form the final drug substance. Thus, the control of stereochemical composition has an important role in the manufacturing process of therapeutic oligonucleotides, either as an in-process control or a final characterization test. In this Perspective, we first present the current status of oligonucleotide therapeutics, with a focus on phosphorothioate derivatives. We then highlight the regulatory framework for controlling the stereochemical composition of these drugs, provide brief insights into the biological significance of stereochemical composition for phosphorothioates, and discuss the origin of stereochemical composition in the synthetic and downstream processes. Subsequently, we provide an in-depth analysis of analytical methods used to determine the stereochemical composition of therapeutic oligonucleotides, including liquid chromatography approaches in various separation modes and emerging modern techniques like ion mobility spectrometry. Furthermore, capillary electrophoresis, duplex melting temperature, and NMR spectroscopy are also discussed in the context of stereochemical composition analysis of therapeutic oligonucleotides. Finally, we highlight novel enzymatic methods for cleaving therapeutic oligonucleotides into smaller fragments that can be analyzed for stereochemical composition using standard techniques.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


