羰基和类羰基化学选择性同源化制备三取代烯基卤化物
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了以酮类、醛类和卤代烷锂为起始原料,化学选择性合成三取代烯烃卤化物(Cl、Br、F、I)。在形成相应的四面体中间加合物后,随后加入亚硫酰氯,触发选择性e2型消除,从而提供目标基序。转化在完全化学控制下进行:各种敏感官能团(例如酯,腈,硝基,卤素基团)可以放置在起始材料上,从而记录了广泛的反应范围,以及该技术在生物活性物质上的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
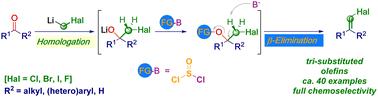
Chemoselective homologative preparation of trisubstituted alkenyl halides from carbonyls and carbenoids†
The chemoselective synthesis of trisubstituted alkenyl halides (Cl, Br, F, I) starting from ketones and aldehydes and lithium halocarbenoids is reported. Upon forming the corresponding tetrahedral intermediate adduct, followed by the addition of thionyl chloride, a selective E2-type elimination is triggered, furnishing the targeted motifs. The transformation takes place under full chemocontrol: various sensitive functionalities (e.g. ester, nitrile, nitro, or halogen groups) can be placed on the starting materials, thus documenting a wide reaction scope, as well as the application of the technique to biologically active substances.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


