乳酸/半胱氨酸双消耗益生菌-纳米药物生物混合系统增强癌症化学免疫治疗
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
免疫治疗是肿瘤学的一场革命,但其治疗效果仍然受到脱靶毒性和较差的抗肿瘤免疫反应的限制。通过将载药纳米颗粒(DMnSH)与小叶细孔菌(Vei)独特的代谢特性相结合,精心设计了一种益生菌-纳米药物偶联物Vei@DMnSH生物杂交物,用于增强癌症化学免疫治疗。具体来说,Vei@DMnSH可以在低氧肿瘤部位积聚,同时消耗乳酸和半胱氨酸,从而逆转乳酸相关的免疫抑制,阻碍GSH的生物合成。此外,DMnSH纳米颗粒会迅速消耗细胞内GSH并分解释放DOX和Mn2+。伴随着GSH双头消耗,Mn2+介导的Fenton-like反应可有效产生氧化羟基自由基,诱导重度氧化还原失衡。结合DOX的治疗效果,激发强大的免疫原性细胞死亡,随后激活抗肿瘤适应性免疫,肿瘤抑制率超过82%,协同提高癌症化学免疫治疗的治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
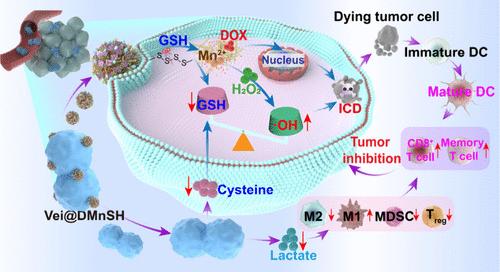
Lactate/Cysteine Dual-Consuming Probiotic–Nanomedicine Biohybrid System for Enhanced Cancer Chemo-Immunotherapy
Immunotherapy is revolutionizing oncology, but its therapeutic efficiency is still limited by the off-target toxicities and poor antitumor immune responses. By integrating the drug-loaded nanoparticles (DMnSH) with the unique metabolic traits of Veillonella parvula (Vei), a probiotic–nanomedicine conjugate Vei@DMnSH biohybrid is elaborately designed for enhanced cancer chemo-immunotherapy. Specifically, Vei@DMnSH can accumulate in hypoxic tumor sites and simultaneously consume lactate and cysteine to reverse the lactate-associated immunosuppression and impede the biosynthesis of GSH. In addition, the DMnSH nanoparticles will rapidly deplete intracellular GSH and disassemble to release DOX and Mn2+. Accompanied by the two-pronged GSH depletion, the Mn2+-mediated Fenton-like reaction can effectively generate oxidative hydroxyl radicals to induce heavy redox imbalance. Combined with the therapeutic effect of DOX, robust immunogenic cell death is provoked and subsequently activates antitumor adaptive immunity with a tumor suppression rate over 82%, synergistically enhancing the therapeutic outcomes of cancer chemo-immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


