dmmt抑制asperaponin VI可减轻GPX4抑制介导的成骨细胞铁下垂和糖尿病性骨质疏松症
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
糖尿病性骨质疏松症(DOP)是糖尿病的一种潜在并发症,治疗选择有限。DOP在病理上与多种类型的受调节细胞死亡相关,但铁下垂在这一过程中的确切作用仍知之甚少。Asperosaponin VI (AVI)以其治疗骨折和骨质疏松症的临床疗效而闻名,其保护骨的作用机制可能与铁下垂有关,但尚未确定。目的探讨AVI在DOP小鼠模型中对铁下垂的调节作用及其机制。方法观察dopo小鼠股骨及原代骨细胞的OP变化。我们培育了一株成骨细胞特异性gpx4缺陷小鼠。采用显微ct、免疫组织化学、免疫荧光、甲基化特异性PCR (MSP)、亚硫酸氢盐测序PCR (BSP)、western blotting (WB)和AVI拉下实验等方法,探讨AVI在DOP中的作用机制和治疗靶点。结果dopo条件小鼠的股骨表现出明显的铁下垂和核心抗铁下垂因子GPX4的抑制,主要是由于DNA甲基转移酶dnmt1和DNMT3a介导的GPX4启动子的超甲基化。值得注意的是,AVI治疗有效地逆转了高甲基化,恢复了GPX4的表达,并通过抑制DNMT1/3a减少了与DOP相关的铁致凋亡病理。在原代成骨细胞中,AVI通过依赖于DNMT抑制和GPX4恢复的机制减轻了dopo条件下的原代成骨细胞的GPX4抑制和铁下沉。重要的是,在成骨细胞Gpx4单倍体缺陷小鼠(Gpx4Ob-/+)或用RSL3药理学灭活Gpx4时,AVI的抗铁和骨保护作用被取消。结论我们的研究发现了一个对DOP有重要作用的关键表观遗传铁致凋亡通路,并揭示了AVI的一个重要药理特性,该特性可能有效治疗DOP及相关骨质疏松症患者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
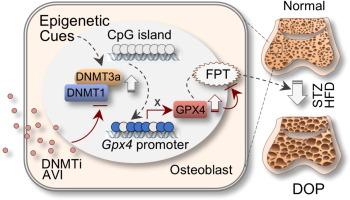
Asperosaponin VI inhibition of DNMT alleviates GPX4 suppression-mediated osteoblast ferroptosis and diabetic osteoporosis
Introduction
Diabetic osteoporosis (DOP) is an insidious complication of diabetes with limited therapeutic options. DOP is pathologically associated with various types of regulated cell death, but the precise role of ferroptosis in the process remains poorly understood. Asperosaponin VI (AVI), known for its clinical efficacy in treating bone fractures and osteoporosis, may exert its osteoprotective effects through mechanisms involving ferroptosis, however this has not been established.Objectives
This study aimed to investigate the role of AVI in modulating ferroptosis in a mouse model of DOP and to explore the underlying mechanisms.Methods
We assessed OP alterations in femurs of DOP-conditioned mice and primary bone cells. We generated a strain of osteoblast-specific Gpx4-deficient mice. A combination of micro-CT, immunohistochemistry, immunofluorescence, methylation-specific PCR (MSP), bisulfite sequencing PCR (BSP), western blotting (WB), and AVI pull-down assays were employed to elucidate the mechanism and therapeutic target of AVI in DOP.Results
Our findings revealed that femurs from DOP-conditioned mice exhibited significant ferroptosis and suppression of the core anti-ferroptosis factor GPX4, mainly due to hypermethylation of the Gpx4 promoter mediated by DNA methyltransferases DNMT1and DNMT3a. Notably, treatment with AVI effectively reversed the hypermethylation, restored GPX4 expression, and reduced ferroptotic pathologies associated with DOP by inhibiting DNMT1/3a. In primary osteoblasts, AVI alleviated GPX4 suppression and reduced ferroptosis in DOP-conditioned primary osteoblasts through a mechanism dependent on DNMT inhibition and GPX4 restoration. Importantly, the anti-ferroptotic and osteoprotective effects of AVI were abolished in osteoblastic Gpx4 haplo-deficient mice (Gpx4Ob-/+) or when GPX4 was pharmacologically inactivated with RSL3.Conclusions
Our study identifies a pivotal epigenetic ferroptotic pathway that contributes significantly to DOP and uncovers a crucial pharmacological property of AVI that is potentially effective in treating patients with DOP and related osteoporotic disorders.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


