第一配位球、第二配位球和远区残基的变异如何影响非血红素Fe(II)/2-氧戊二酸依赖的乙烯形成酶的催化机制?
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
乙烯形成酶(EFE)是一种铁(II)/2-氧戊二酸酯(2OG)和l-精氨酸(l-Arg)依赖的加氧酶,主要将2OG分解成乙烯,同时也催化l-Arg羟基化。虽然EFE中的羟基化机制与其他依赖Fe(II)/ 2g的加氧酶相似,但乙烯的形成是独特的。各种重新设计策略旨在提高EFE的乙烯产量,但成功有限,突出了替代方法的必要性。对蛋白质环境的综合和多维效应进行准确和全面的描述对于加强金属酶,特别是EFE的再设计策略至关重要。这包括了解第二配位球(SCS)和远程(LR)相互作用残基,相关运动,电子结构,本征电场(IntEF)的作用,以及过渡态和反应中间体的稳定。在这项研究中,我们采用基于分子动力学的量子力学/分子力学方法来研究蛋白质环境对第一配位球EFE变体(FCS, D191E), SCS (A198V和R171A)和LR (E215A)催化反应的综合影响。该研究揭示了EFE中不同位置的取代如何相似地影响乙烯生成反应,同时对羟基化反应产生不同的影响。结果预测了变异在控制Fe(II)中心的2OG配位模式中的作用。具体来说,该研究表明D191E独特地倾向于在双氧结合之前从离线到在线的2OG协调模式过渡。然而,对D191E突变体中存在离线接近双氧和双氧结合时的2OG翻转的研究表明,在5C Fe(II)状态下,2OG翻转可能不可行。计算表明,在EFE及其变体(D191E除外)中存在氢原子转移(HAT)辅助氧翻转的可能性。MD模拟揭示了D191E突变体α7区的特征动态变化,这可能有助于其羟基化反应的增强。结果表明,在D191E突变体中,IM2 (Fe(III)-部分键中间体)可能形成一个在线铁基。这种来自IM2的替代途径也可能存在于WT、EFE和其他变体中,这些变体尚未被探索。研究还描述了取代对电子结构和interf的影响。总的来说,计算支持这样的观点,即理解蛋白质环境对EFE变体催化反应的综合和多维影响,为改进EFE的酶重新设计方案以增加乙烯产量提供了基础。本研究的结果也将有助于开发其他金属酶的替代重新设计策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
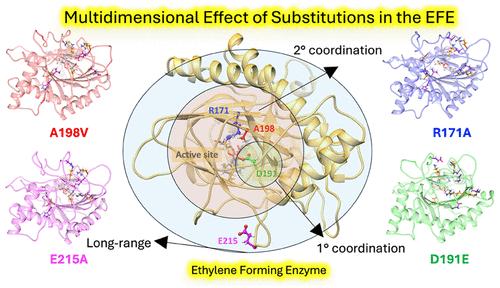
How Do Variants of Residues in the First Coordination Sphere, Second Coordination Sphere, and Remote Areas Influence the Catalytic Mechanism of Non-Heme Fe(II)/2-Oxoglutarate Dependent Ethylene-Forming Enzyme?
The ethylene-forming enzyme (EFE) is a Fe(II)/2-oxoglutarate (2OG) and l-arginine (l-Arg)-dependent oxygenase that primarily decomposes 2OG into ethylene while also catalyzing l-Arg hydroxylation. While the hydroxylation mechanism in EFE is similar to other Fe(II)/2OG-dependent oxygenases, the formation of ethylene is unique. Various redesign strategies have aimed to increase ethylene production in EFE, but success has been limited, highlighting the need for alternate approaches. It is crucial to incorporate an accurate and comprehensive description of the integrative and multidimensional effects of the protein environment to enhance the redesign strategy in metalloenzymes, particularly in EFE. This involves understanding the role of the second coordination sphere (SCS) and long-range (LR) interacting residues, correlated motions, electronic structure, intrinsic electric field (IntEF), as well as the stabilization of transition states and reaction intermediates. In this study, we employ a molecular dynamics-based quantum mechanics/molecular mechanics approach to examine the integrative effects of the protein environment on reactions catalyzed by EFE variants from the first coordination sphere (FCS, D191E), SCS (A198V and R171A) and LR (E215A). The study uncovers how substitutions at different positions in EFE similarly impact the ethylene-forming reaction while posing distinct effects on the hydroxylation reaction. Results predict the effect of the variants in controlling the 2OG coordination mode in the Fe(II) center. Specifically, the study suggests that D191E uniquely prefers transitioning from an off-line to an in-line 2OG coordination mode before dioxygen binding. However, studies on the 2OG flip in the presence of off-line approaching dioxygen and dioxygen binding in the D191E variant indicate that the 2OG flip might not be feasible in the 5C Fe(II) state. Calculations show the possibility of a hydrogen atom transfer (HAT)-assisted oxygen flip in EFE and its variants (other than D191E). MD simulations elucidate the characteristic dynamic change in the α7 region in the D191E variant that might contribute to its increased hydroxylation reaction. Results indicate the possibility of forming an in-line ferryl from the IM2 (Fe(III)-partial bond intermediate) in the D191E variant. This alternative pathway from IM2 may also exist in WT EFE and other variants, which are yet to be explored. The study also delineates the impact of substitutions on the electronic structure and IntEF. Overall, the calculations support the idea that understanding the integrative and multidimensional effects of the protein environment on the reactions catalyzed by EFE variants provides the basics for improved enzyme redesign protocols of EFE to increase ethylene production. The results of this study will also contribute to the development of alternate redesign strategies for other metalloenzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


