完全生物内皮化组织工程血管导管具有抗血栓功能和移植物通畅
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
组织工程血管导管(tevc)通常是通过将自体骨髓细胞植入可生物降解的聚合物支架而制成的,有望用于治疗单心室先天性心脏缺陷(SVCHDs)。然而,在先前的TEVC临床试验中,移植物狭窄的高发生率阻碍了TEVC的临床应用。在此,我们在流动生物反应器中,将人诱导多能干细胞(hiPSC)来源的内皮细胞(ECs)涂覆在去细胞化的人脐动脉管腔表面,然后进行剪切应力训练,从而开发出内皮化的tevc。这些tevc在裸鼠植入下腔静脉后具有立即的抗血栓功能,并加速宿主EC的募集。移植物保持通畅,无血栓形成,随后完全替换宿主ECs。我们的研究为未来生产由hipsc衍生的ECs组成的全生物tevc作为SVCHDs的创新疗法奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
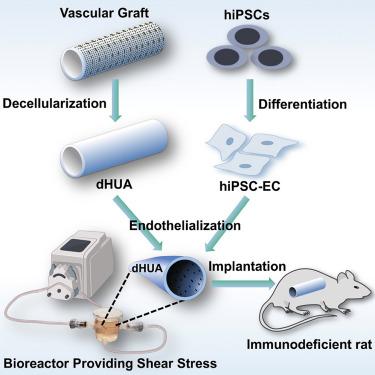
Fully biologic endothelialized-tissue-engineered vascular conduits provide antithrombotic function and graft patency
Tissue-engineered vascular conduits (TEVCs), often made by seeding autologous bone marrow cells onto biodegradable polymeric scaffolds, hold promise toward treating single-ventricle congenital heart defects (SVCHDs). However, the clinical adoption of TEVCs has been hindered by a high incidence of graft stenosis in prior TEVC clinical trials. Herein, we developed endothelialized TEVCs by coating the luminal surface of decellularized human umbilical arteries with human induced pluripotent stem cell (hiPSC)-derived endothelial cells (ECs), followed by shear stress training, in flow bioreactors. These TEVCs provided immediate antithrombotic function and expedited host EC recruitment after implantation as interposition inferior vena cava grafts in nude rats. Graft patency was maintained with no thrombus formation, followed by complete replacement of host ECs. Our study lays the foundation for future production of fully biologic TEVCs composed of hiPSC-derived ECs as an innovative therapy for SVCHDs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


