肺炎球菌肺炎是由细菌素介导的菌株内竞争引起的细菌周转增加引起的
IF 5.1
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
利用染色体条形码,我们观察到97%的肺炎链球菌(Spn)在小鼠模型中接种后2天内在肺部翻转。这种明显的多样性崩溃和细菌更替与急性炎症(严重的肺炎球菌肺炎)、肺部细菌数量高、菌血症和死亡率有关。这些效应都需要由blp位点介导的菌株内竞争,blp位点以群体感应依赖的方式表达细菌素。由blp -细菌素活性引起的细菌周转增加了肺炎球菌毒素溶肺素(Ply)的释放,这足以解释肺部病理。Ply逃避补体的能力,而不是其成孔活性,阻止了Spn的调性自噬细胞清除,使其在肺中增殖,促进炎症反应和随后的侵入血流。因此,我们的研究证明了在感染过程中对细菌种群动态的欣赏如何为发病机制提供新的见解。对感染期间肺炎球菌群体结构的分析揭示了细菌素通过促进炎症介质的释放在推动肺炎发展中的未被认识的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
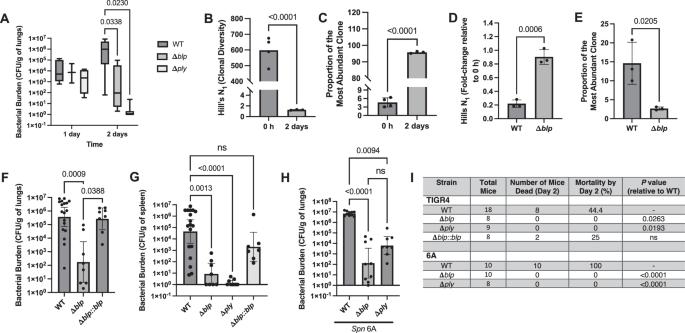
Pneumococcal pneumonia is driven by increased bacterial turnover due to bacteriocin-mediated intra-strain competition
Using chromosomal barcoding, we observed that >97% of the Streptococcus pneumoniae (Spn) population turns over in the lung within 2 days post-inoculation in a murine model. This marked collapse of diversity and bacterial turnover was associated with acute inflammation (severe pneumococcal pneumonia), high bacterial numbers in the lungs, bacteremia, and mortality. Intra-strain competition mediated by the blp locus, which expresses bacteriocins in a quorum-sensing-dependent manner, was required for each of these effects. Bacterial turnover from the activity of Blp-bacteriocins increased the release of the pneumococcal toxin, pneumolysin (Ply), which was sufficient to account for the lung pathology. The ability of Ply to evade complement, rather than its pore-forming activity, prevented opsonophagocytic clearance of Spn enabling its multiplication in the lung, facilitating the inflammatory response and subsequent invasion into the bloodstream. Thus, our study demonstrates how an appreciation for bacterial population dynamics during infection provides new insight into pathogenesis. Profiling of pneumococcal population structure during infection reveals an unappreciated role of bacteriocins in driving the development of pneumonia by facilitating the release of inflammatory mediators.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


