巯基和双氧水催化炔烃的z选择性氢亚砜化反应
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
虽然亚砜是生物分子中重要的结构基序,也是合成化学中普遍存在的原料,但由于Z型几何结构的热力学不稳定性和金属催化剂中硫亲核试剂的毒性,寻找(Z)-α,β-不饱和亚砜的通用和可持续的途径仍然具有很大的挑战性。在这里,我们报告了一种基于均相八胺酸酯催化的抗氢硫化和Z保留亚砜化方法的新方案,允许从市售炔、硫醇和H2O2中获得(Z)-α,β-不饱和亚砜。对照实验研究表明,钼分子是硫基自由基引发剂、z型几何介质和亚砜化催化剂。值得注意的是,该一锅反应具有底物范围广、立体选择性高(Z/E高达 >; 99:1)和反应条件温和的特点,为制备Z-乙烯基亚砜化合物提供了一条很有前景的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
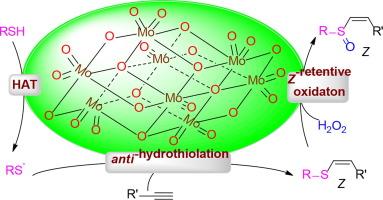

Z-Selective hydrosulfoxidation of alkynes with thiols and hydrogen peroxide Enabled by a Multifunctional octamolybdate
Although sulfoxide is an important structural motif found in biological molecules and prevalent feedstocks in synthetic chemistry, a general and sustainable access route to (Z)-α,β-unsaturated sulfoxides remains highly challenging due to the thermodynamic instability of Z-type geometric and the notorious sulfur nucleophiles poisoning of metal catalyst. Here we report a new protocol based on homogeneous octamolybdate-catalyzed anti-hydrothiolation and after Z-retentive sulfoxidation methodology, allowing access to (Z)-α,β-unsaturated sulfoxides from commercially available alkynes, thiols and H2O2. Control experiment investigations indicate that the molecular molybdenum is a thiyl radical initiator, Z-type geometric mediator and sulfoxidation catalyst. Notably, this one-pot reaction features broad substrate scopes with high stereoselectivity (Z/E up to > 99:1) and mild reaction conditions, thus providing a promising approach for the preparation of Z-vinyl sulfoxide compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


