微生物源性短链脂肪酸决定了胃主细胞的干细胞特征
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
胃黏膜是一个高度动态的组织,通过干细胞分化不断自我更新。主细胞在稳态中保持静止状态,但在损伤后负责再生。虽然微生物群-宿主相互作用在肠道中的作用研究得很好,但对这些相互作用在胃中的作用知之甚少。通过小鼠类器官和无菌小鼠模型,我们发现微生物源性短链脂肪酸(SCFAs)抑制小鼠主细胞的增殖。这种作用是通过激活g蛋白偶联受体43介导的。最重要的是,通过代谢组学和移植研究,我们发现产生丁酸盐的肠乳杆菌调节小鼠主细胞的增殖。我们的研究结果确定了微生物群调节主细胞细胞特征的机制,为宿主与其微生物环境之间的复杂相互作用以及胃内稳态的潜在机制提供了深入的了解,对胃疾病具有潜在的治疗意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
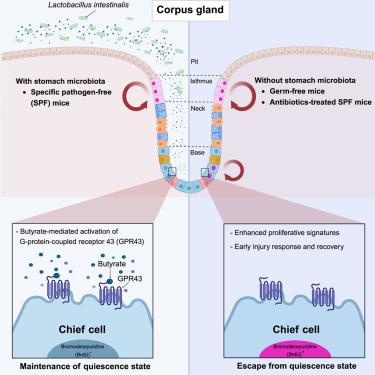
Microbiota-derived short-chain fatty acids determine stem cell characteristics of gastric chief cells
The gastric mucosa is a highly dynamic tissue that undergoes constant self-renewal through stem cell differentiation. Chief cells maintain a quiescent state in homeostasis but are responsible for regeneration after injury. Although the role of microbiome-host interactions in the intestine is well studied, less is known about these interactions in the stomach. Using the mouse organoid and germ-free mouse models, we show that microbiota-derived short-chain fatty acids (SCFAs) suppress the proliferation of chief cells in mice. This effect is mediated by activation of G-protein-coupled receptor 43. Most importantly, through metabolomics and transplantation studies, we show butyrate-producing Lactobacillus intestinalis modulates the proliferation of chief cells in mice. Our findings identify a mechanism by which the microbiota regulates the cell characteristics of chief cells, providing insight into the complex interplay between the host and its microbial environment and the mechanisms underlying gastric homeostasis, with potential therapeutic implications for gastric diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


