低渗透性通过重塑细胞骨架和增加染色质可及性来促进原始多能性
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
细胞命运的决定和转变在生物学和医学中是至关重要的。原始多能性可以通过对分化细胞进行重编程来实现。然而,其机制尚不清楚。渗透压是作用于活细胞,尤其是多能细胞的一个重要物理因素,但其在细胞命运转变中的意义尚不清楚。目的探讨渗透压在细胞命运转变中的作用及其机制。方法采用流式细胞术、定量PCR、畸胎瘤和嵌合小鼠实验来评估iPSCs的重编程效率和特性。利用透射电镜(TEM)、免疫荧光染色、免疫印迹(western blot)、化学处理和基因修饰等方法观察细胞形态、信号通路、细胞骨架和细胞核结构。应用多组测序揭示EpiSCs在低渗透条件下的转录组、组蛋白标记和染色质可及性。结果在低渗透条件下,低渗透EpiSCs的重编程效率(1100 vs 18菌落/ 3 × 105细胞)比等渗透细胞高60倍以上,而在高渗透细胞中未形成菌落。转化的细胞显示出初始多能性。低渗透EpiSCs表现出较大的细胞大小、核面积和较少的异染色质;ATAC-seq和ChIP-seq证实了具有更多H3K27ac的原始多能基因位点的可及性增加。从机制上讲,低渗透压激活EpiSCs中的PI3K-AKT-SP1信号,从而重塑细胞骨架和核骨架,导致基因组重组和多能基因表达。相反,低渗透压延迟了ESCs从初始多能性的退出。此外,在mef重编程中,低渗透压促进了向初始多能性的转化。结论低渗透压通过PI3K-AKT-SP1通路重塑细胞骨架、核骨架和基因组,促进细胞命运转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
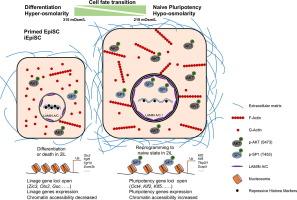
Hypo-osmolarity promotes naive pluripotency by reshaping cytoskeleton and increasing chromatin accessibility
Introduction
Cell fate determination and transition are of paramount importance in biology and medicine. Naive pluripotency could be achieved by reprogramming differentiated cells. However, the mechanism is less clear. Osmolarity is an essential physical factor that acts on living cells, especially for pluripotent cells, but its significance in cell fate transition remains unexplored.Objectives
To investigate the role of osmolarity in cell fate transition and its underlying mechanism.Methods
Flow cytometry, quantitative PCR, teratoma and chimeric mice assays were performed to assess reprogramming efficiency and characterize iPSCs. TEM, immunofluorescence staining, western blot, chemical treatment and genetic modification were utilized to evaluate cell morphology, signaling pathways, cytoskeleton and nuclear structure. Multiomic sequencings were applied to unveil the transcriptome, histone markers and chromatin accessibility of EpiSCs in hypo-osmotic condition.Result
In hypo-osmotic condition, the reprogramming efficiency of hypo-osmotic EpiSCs increased over 60-fold than that of iso-osmotic cells (1100 vs 18 colonies per 3 × 105 cells), whereas no colony formed in hyper-osmotic cells. The converted cells displayed naive pluripotency. The hypo-osmotic EpiSCs exhibited larger cell size, nuclear area and less heterochromatin; ATAC-seq and ChIP-seq confirmed the increased accessibility of naive pluripotent gene loci with more H3K27ac. Mechanistically, hypo-osmolarity activated PI3K-AKT-SP1 signaling in EpiSCs, which reshaped cytoskeleton and nucleoskeleton, resulting in genome reorganization and pluripotent gene expression. In contrast, hypo-osmolarity delayed the ESCs’ exit from naive pluripotency. Moreover, in MEFs reprograming, hypo-osmolarity promoted the conversion to naive pluripotency.Conclusion
Hypo-osmolarity promotes cell fate transition by remodeling cytoskeleton, nucleoskeleton and genome via PI3K-AKT-SP1 pathway.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


