人类成人神经发生丧失与癫痫进展过程中的认知能力下降相对应
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
内侧颞叶癫痫(MTLE)是一种以癫痫发作和认知合并症为表现的综合征性疾病。虽然癫痫的病因越来越被了解,但导致认知能力下降和癫痫进展的病理生理机制仍然不太清楚。我们之前的研究表明,随着病程的延长,MTLE患者的成年海马神经发生显著下降。在这里,我们研究当多个认知领域在癫痫进展过程中受到影响,以及人类神经发生水平如何促进它。我们发现,智力、语言学习和记忆力在疾病持续20年的关键时期下降。与啮齿类动物相比,人类未成熟神经元的数量与听觉、语言、学习和记忆呈正相关,而不是视觉空间。此外,这种关联并不适用于成熟的颗粒神经元。我们的研究提供了成人神经发生如何与人类认知相对应的细胞证据,并标志着推进MTLE和其他认知障碍患者的再生医学的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
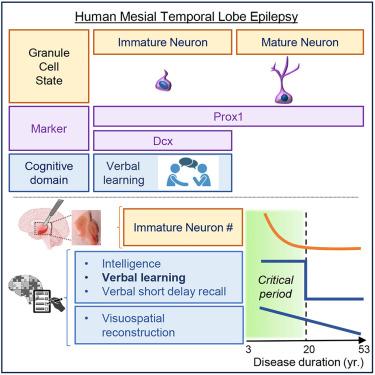
Human adult neurogenesis loss corresponds with cognitive decline during epilepsy progression
Mesial temporal lobe epilepsy (MTLE) is a syndromic disorder presenting with seizures and cognitive comorbidities. Although seizure etiology is increasingly understood, the pathophysiological mechanisms contributing to cognitive decline and epilepsy progression remain less recognized. We have previously shown that adult hippocampal neurogenesis dramatically declines in MTLE patients with increased disease duration. Here, we investigate when multiple cognitive domains become affected during epilepsy progression and how human neurogenesis levels contribute to it. We find that intelligence, verbal learning, and memory decline at a critical period of 20 years disease duration. In contrast to rodents, the number of human immature neurons positively associates with auditory verbal, rather than visuospatial, learning and memory. Moreover, this association does not apply to mature granule neurons. Our study provides cellular evidence of how adult neurogenesis corresponds with human cognition and signifies an opportunity to advance regenerative medicine for patients with MTLE and other cognitive disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


