通过Suzuki-Miyaura交叉偶联反应研究钯(II)预催化剂中的芳酰甲酰胺配体
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了氧化还原活性芳唑甲酰胺(AAF)配体在Suzuki-Miyaura交叉偶联反应钯(II)预催化剂中的合成和应用。在合适的溶剂中,AAF配体与Pd(II)Cl2形成2等量的配合物,形成方形平面(AAF)2PdCl2预催化剂。对芳基溴化物和芳基硼酸的深入研究发现,1.0 mol %的预催化剂,以碳酸铯(Cs2CO3)为碱,1,4-二氧六环为溶剂,在90°C下反应24小时,可以很好地转化为联苯产品(超过20个例子)。为了突出AAF配体的类别,我们与具有氧化还原活性的芳唑硫代甲酰胺配体进行了一系列比较反应,即(ATF)2PdCl2配合物、其他商业钯(II)配合物和Ni(II)Cl2芳唑甲酰胺配位配合物2。(AAF)2PdCl2配合物优于所有其他测试。在机理上,提出了AAF配体作为金属转化中间体与钯(I)配合物单还原为反铁磁偶联。镍基预催化剂对所研究的Suzuki-Miyaura反应无活性。总的来说,这些配体系统为氧化还原活性钯交叉偶联反应以及无膦和高产提供了独特的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
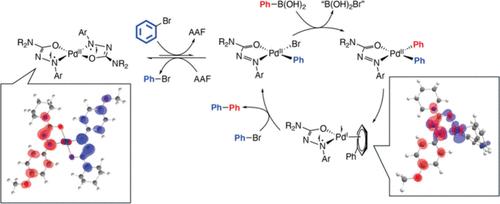
Investigating Arylazoformamide Ligands in Palladium(II) Precatalysts through the Suzuki–Miyaura Cross-Coupling Reaction
Herein, is the reported synthesis and utilization of redox-active arylazoformamide (AAF) ligands in palladium(II) precatalysts for the Suzuki–Miyaura cross-coupling reaction. Complexes were formed from 2 equiv of an AAF ligand with Pd(II)Cl2 in an appropriate solvent to create the square planar (AAF)2PdCl2 precatalyst. A thorough investigation of aryl bromides and arylboronic acids found that 1.0 mol % precatalyst with cesium carbonate (Cs2CO3) as base, 1,4-dioxane as solvent at 90 °C for 24 h allowed for excellent conversions to the biphenyl products (over 20 examples). To highlight the AAF ligand class, a set of comparison reactions were performed with redox-active arylazothioformamide ligands, i.e., an (ATF)2PdCl2 complex, other commercial palladium(II) complexes, and a Ni(II)Cl2 arylazoformamide coordination complex2. The (AAF)2PdCl2 complexes outperformed all of others tested. Mechanistically, it is proposed that the AAF ligand singly reduces to antiferromagnetically couple to the palladium(I) complex as a transmetalation intermediate. Ni-based precatalysts were found to be inactive for the studied Suzuki–Miyaura reaction. Overall, these ligand systems offer a unique look into redox-active palladium cross-coupling reactions as well as being phosphine-free and high yielding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


