磁性可回收CoFe2O4纳米催化剂用于聚对苯二甲酸乙二醇酯的高效糖酵解
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
高效和可持续地回收聚对苯二甲酸乙二醇酯(PET)对于减轻其对气候、人类健康和全球生态系统的环境影响至关重要。糖酵解是一种将PET转化为双(2-羟乙基)对苯二甲酸酯(BHET)的闭环回收方法,由于其温和的操作条件和环保性而成为最有前途的方法之一。然而,将PET完全转化为具有高稳定性的BHET仍然具有挑战性。本研究采用溶剂热法制备了磁可回收的CoFe2O4纳米催化剂,并以Na3Cit·2H2O为改性剂进行了表面调制。当使用优化的CoFe2O4催化剂时,PET的转化率达到100 %,在210 °C下,1 h, BHET的收率为91.7 %。CoFe2O4优异的催化性能是由于其粒径更小,分散性更好,表面Co/Fe比更高,氧空位增加,所有这些都可以通过直接的表面调制来实现。M−O距离(M = Co或Fe)、吸附能和Bader电荷的DFT计算证实,较高的表面Co/Fe比增强了PET糖酵解,与实验结果一致。此外,还提出了一个修正的能量经济系数(εm)来表征催化效率。CoFe2O4-60 %的εm值为0.624,在PET的高效糖酵解中具有广阔的应用前景。这项工作提出了一种通用的方法,可以轻松地操纵磁性催化剂的表面性质,并确定了实现PET-to-BHET转化高性能的关键因素。这为未来设计具有磁性的纳米颗粒化学催化催化剂提供了有价值的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
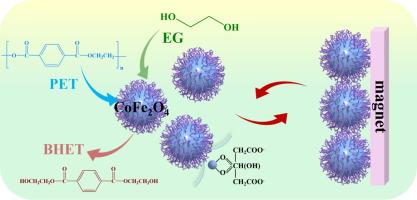
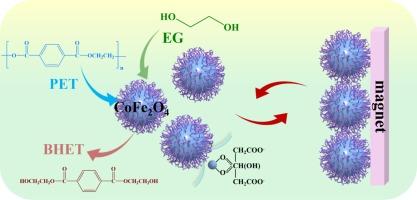
Magnetically recyclable CoFe2O4 nanocatalysts for efficient glycolysis of polyethylene terephthalate
Efficient and sustainable recycling of polyethylene terephthalate (PET) is essential in mitigating its environmental impacts on climate, human health, and global ecosystems. Glycolysis, a closed-loop recycling method that converts PET into bis(2-hydroxyethyl) terephthalate (BHET), stands out as one of the most promising methods due to its mild operating conditions and environmentally friendly nature. However, the complete convert PET into BHET with a high stability are still challenging. In this study, magnetically recyclable CoFe2O4 nanocatalysts were synthesized by the solvothermal method and surface-modulated with Na3Cit·2H2O as a modifier. When utilizing an optimized CoFe2O4 catalyst, conversion of PET achieved 100 %, with a BHET yield of 91.7 % at 210 °C for 1 h. The excellent catalytic performance of CoFe2O4 is attributed to its smaller particle size, improved dispersion, higher surface Co/Fe ratio, and increased oxygen vacancies, all of which can be achieved through straightforward surface modulation. DFT calculations of M−O distance (M = Co or Fe), adsorption energy, and Bader charges confirm that a higher surface Co/Fe ratio enhanced PET glycolysis, consistent with experimental results. Additionally, a modified energy economy coefficient (εm) was proposed to characterize the catalytic efficiency. The εm value of CoFe2O4-60 % was 0.624, indicating promising applications in efficient PET glycolysis. This work presents a versatile approach for easily manipulating the surface properties of magnetic catalysts and identifies key factors for achieving high performance in PET-to-BHET conversion. It offers valuable guidelines for the future design of nanoparticle catalysts with magnetic properties for chemocatalytic reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


