对生物膜结构和成熟的洞察使体外多糖靶向治疗的临床策略得到改善
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
多糖胞间黏附素(PIA)是一种由聚n -乙酰氨基葡萄糖(PNAG)组成的胞外多糖,是许多致病性生物膜的重要组成部分。PNAG的部分去乙酰化是生物膜形成所必需的,但有限的结构知识阻碍了治疗的发展。利用一种新的单克隆抗体(TG10)选择性结合高度去乙酰化的PNAG和一种抗体(F598)在临床试验中结合高度乙酰化的PNAG,我们证明了生物膜内的PIA含有不同的高度乙酰化和去乙酰化的外多糖区域,这与之前在整个生物膜中随机去乙酰化的模型相反。这一发现使我们假设,针对两种形式的PNAG将提高疗效。值得注意的是,TG10和F598协同提高了体外和体内活性,在致死性金黄色葡萄球菌攻击小鼠模型中提供了90%的存活率。我们的先进模型加深了对PIA结构和成熟的概念理解,并揭示了针对PIA的治疗方法、疫苗和诊断试剂的改进设计策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

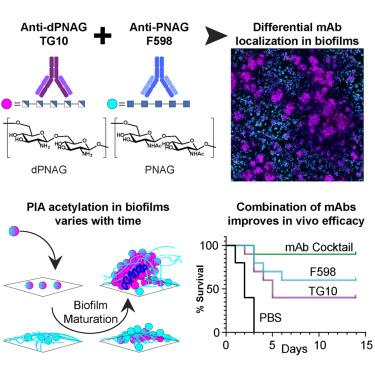
Insights into biofilm architecture and maturation enable improved clinical strategies for exopolysaccharide-targeting therapeutics
Polysaccharide intercellular adhesin (PIA), an exopolysaccharide composed of poly-N-acetyl glucosamine (PNAG), is an essential component in many pathogenic biofilms. Partial deacetylation of PNAG is required for biofilm formation, but limited structural knowledge hinders therapeutic development. Employing a new monoclonal antibody (TG10) that selectively binds highly deacetylated PNAG and an antibody (F598) in clinical trials that binds highly acetylated PNAG, we demonstrate that PIA within the biofilm contains distinct regions of highly acetylated and deacetylated exopolysaccharide, contrary to the previous model invoking stochastic deacetylation throughout the biofilm. This discovery led us to hypothesize that targeting both forms of PNAG would enhance efficacy. Remarkably, TG10 and F598 synergistically increased in vitro and in vivo activity, providing 90% survival in a lethal Staphylococcus aureus challenge murine model. Our advanced model deepens the conceptual understanding of PIA architecture and maturation and reveals improved design strategies for PIA-targeting therapeutics, vaccines, and diagnostic agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


