含氯二氟烷烃的pd催化烯烃二氟烷基化反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
宝石-二氟乙烯单元(- CF2 -)在扩大化学空间方面发挥着关键作用,因为它能够诱导独特的分子几何形状和物理化学性质,使其在制药、农用化学品和先进材料的开发中非常理想。尽管具有重要意义,但将−CF2 -基团整合到有机分子中的有效和通用方法仍然具有挑战性。我们通过正式的Mizoroki-Heck反应描述了钯催化的烯烃二氟烷基化,提供了一种简单、经济、原子经济的途径,从现成的烯烃和氯二氟烷烃合成多种rcf2 -烯烃。该方法有助于在温和条件下将宝石-二氟乙烯基团结合到复杂分子中,并表现出显著的官能团耐受性,从而实现后期功能化。初步机理研究支持钯催化的杂化自由基-极性交叉机制。这种方法提供了一种高效和选择性的方法来整合二氟乙烯单元,扩大了设计生物活性分子和先进材料的机会,并推进了各种应用的合成策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
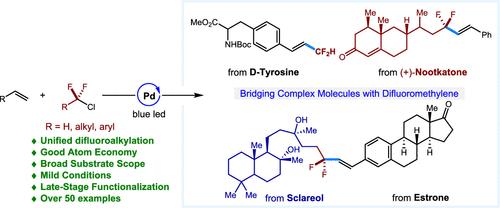
Pd-Catalyzed Difluoroalkylation of Alkenes Using Chlorodifluoroalkanes
The gem-difluoromethylene unit (−CF2−) plays a pivotal role in expanding chemical space due to its ability to induce unique molecular geometries and physicochemical properties, making it highly desirable in the development of pharmaceuticals, agrochemicals, and advanced materials. Despite their significance, efficient and versatile methods for incorporating −CF2– groups into organic molecules remain challenging. We describe a palladium-catalyzed difluoroalkylation of alkenes via a formal Mizoroki–Heck reaction, providing a straightforward, cost-effective, and atom-economic route to synthesize a diverse array of RCF2-alkenes from readily available alkenes and chlorodifluoroalkanes. This method facilitates the incorporation of gem-difluoromethylene groups into complex molecules under mild conditions and demonstrates remarkable functional group tolerance, enabling late-stage functionalization. Preliminary mechanistic studies support a hybrid palladium-catalyzed radical-polar crossover mechanism. This approach offers an efficient and selective means to incorporate difluoromethylene units, broadening opportunities for the design of bioactive molecules and advanced materials and advancing synthetic strategies for a variety of applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


