双靶向仿生脂质体系统阻断肿瘤-星形胶质细胞间隙连接以减轻化疗耐药和治疗脑转移
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
脑转移是各种恶性肿瘤发病率和死亡率的重要组成部分,其特点是具有高度的化疗耐药性。癌细胞和星形胶质细胞之间通过间隙连接的细胞内通信,主要由连接蛋白43组装,已被证明在这一过程中发挥重要作用。然而,有效阻断两种细胞类型之间的间隙连接仍然极具挑战性,因为药物递送不足。在此,我们设计了一个连接蛋白阻断剂-卡贝诺洛酮(CBX)负载的仿生脂质体系统,将人工脂质体与脑转移细胞和反应性星形胶质细胞膜(LAsomes)融合,以阻断间隙连接并减轻化学耐药。lasome通过信号素4D (SEMA 4D)─丛状蛋白B1的相互作用有效地穿透血脑屏障,并通过同型识别主动迁移到它们的源细胞。因此,LAsomes有效地抑制转移细胞通过间隙连接向星形胶质细胞的物质转移和Ca2+流动,从而显著增加转移肿瘤细胞对化疗的敏感性。这些结果表明,关闭间隙连接可能是治疗顽固性脑转移的一种有希望的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
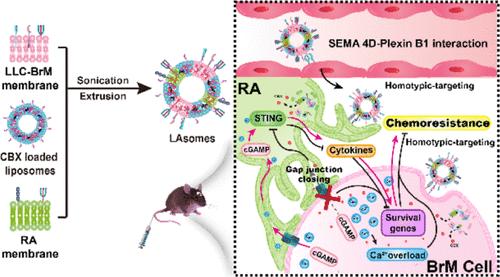
Carcinoma–Astrocyte Gap Junction Interruption by a Dual-Targeted Biomimetic Liposomal System to Attenuate Chemoresistance and Treat Brain Metastasis
Brain metastasis contributes substantially to the morbidity and mortality of various malignancies and is characterized by high chemoresistance. Intracellular communication between carcinoma cells and astrocytes through gap junctions, which are assembled mainly by the connexin 43 protein, has been shown to play a vital role in this process. However, effectively blocking the gap junctions between the two cell types remains extremely challenging because of insufficient drug delivery to the target site. Herein, we designed a connexin blocker-carbenoxolone (CBX)-loaded biomimetic liposomal system with artificial liposomes fused with brain metastatic cell and reactive astrocyte membranes (LAsomes) to block gap junctions and attenuate chemoresistance. LAsomes effectively penetrated the blood–brain barrier via semaphorin 4D (SEMA 4D)─Plexin B1 interactions and actively migrated to their source cells via homotypic recognition. Consequently, LAsomes effectively inhibited material transfer and Ca2+ flow from metastatic cells to astrocytes via gap junctions, thereby markedly increasing the sensitivity of metastatic tumor cells to chemotherapy. These results reveal that closing the gap junctions may be a promising therapeutic strategy for intractable brain metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


