在耻垢分枝杆菌中,ClpS用初级不稳定残基指导n -降解底物
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
耐药结核病感染是对全球公共卫生的重大威胁。必不可少的分枝杆菌ClpC1P1P2蛋白酶作为新型抗菌药物的潜在靶点受到关注。然而,由于我们对其生理蛋白水解的知识有限,探索其在细胞中的功能的努力受到了限制。在这里,我们研究了分枝杆菌ClpS在指导耻垢分枝杆菌ClpC1P1P2进行N-degron途径蛋白水解中的作用。结合实验表明,分枝杆菌ClpS以中等亲和力结合典型的初级不稳定残基(Leu, Phe, Tyr, Trp)。N-degron结合限制了ClpS N-degron结合口袋附近环的构象灵活性,并将ClpS•ClpC1结合亲和力增强了30倍,为细胞在底物丰富时优先考虑N-degron蛋白水解提供了一种机制。对耻毛分枝杆菌的蛋白水解报告试验证实了含有初级N-degrons的底物的降解,但表明分枝杆菌中没有次级N-degrons。这项工作扩展了我们对分枝杆菌N-degron途径的理解,并确定ClpS是底物特异性的关键成分,为开发改进的Clp蛋白酶抑制剂提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
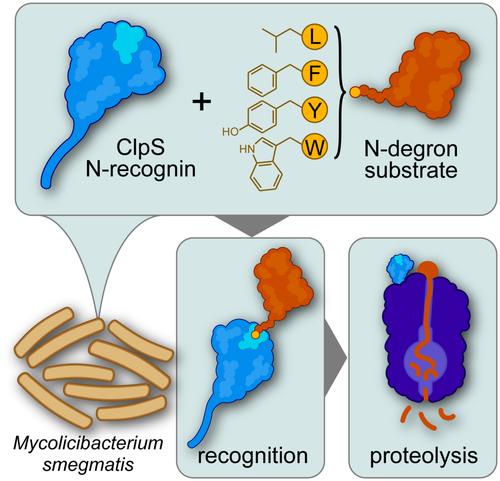
ClpS Directs Degradation of N-Degron Substrates With Primary Destabilizing Residues in Mycolicibacterium smegmatis
Drug-resistant tuberculosis infections are a major threat to global public health. The essential mycobacterial ClpC1P1P2 protease has received attention as a prospective target for novel antibacterial therapeutics. However, efforts to probe its function in cells are constrained by our limited knowledge of its physiological proteolytic repertoire. Here, we interrogate the role of mycobacterial ClpS in directing N-degron pathway proteolysis by ClpC1P1P2 in Mycolicibacterium smegmatis. Binding assays demonstrate that mycobacterial ClpS binds canonical primary destabilizing residues (Leu, Phe, Tyr, Trp) with moderate affinity. N-degron binding restricts the conformational flexibility of a loop adjacent to the ClpS N-degron binding pocket and strengthens ClpS•ClpC1 binding affinity ~30-fold, providing a mechanism for cells to prioritize N-degron proteolysis when substrates are abundant. Proteolytic reporter assays in M. smegmatis confirm degradation of substrates bearing primary N-degrons, but suggest that secondary N-degrons are absent in mycobacteria. This work expands our understanding of the mycobacterial N-degron pathway and identifies ClpS as a critical component for substrate specificity, providing insights that may support the development of improved Clp protease inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


