热腐细胞尸体顶部有富含f -肌动蛋白的丝状伪足,参与传入树突状细胞的CLEC9A信号
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
细胞凋亡破坏细胞以加强免疫沉默,而热噬细胞死亡则引发炎症。很少有人知道细胞的结构结构经历焦亡,以及焦亡尸体是否免疫原性。在本研究中,我们报道了在热噬细胞破裂前几分钟,炎性小体触发气真皮蛋白-d和钙依赖的丝状足从质膜上喷发出来,从而使丝状足形成尸体。作为f -肌动蛋白的丰富储存,焦噬丝状足通过f -肌动蛋白受体CLEC9A (DNGR1)被树突状细胞识别。我们建议细胞在细胞破裂前聚集丝状足,作为死后标记,用于死于气真皮蛋白诱导的焦亡或mlkl诱导的坏死,以供树突状细胞识别。这项研究揭示了焦亡的壮观形态,并确定了炎症小体诱导焦亡细胞构建一个新的警报蛋白的机制,该警报蛋白通过CLEC9A激活树突状细胞,协调从先天免疫到适应性免疫的转变1,2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
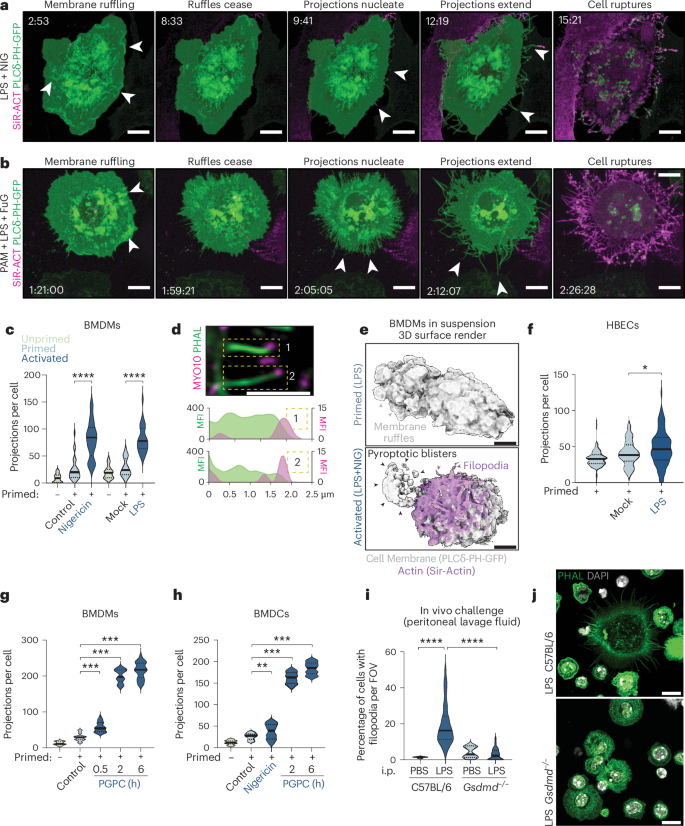

Pyroptotic cell corpses are crowned with F-actin-rich filopodia that engage CLEC9A signaling in incoming dendritic cells
While apoptosis dismantles the cell to enforce immunological silence, pyroptotic cell death provokes inflammation. Little is known of the structural architecture of cells undergoing pyroptosis, and whether pyroptotic corpses are immunogenic. Here we report that inflammasomes trigger the Gasdermin-D- and calcium-dependent eruption of filopodia from the plasma membrane minutes before pyroptotic cell rupture, to crown the resultant corpse with filopodia. As a rich store of F-actin, pyroptotic filopodia are recognized by dendritic cells through the F-actin receptor, CLEC9A (DNGR1). We propose that cells assemble filopodia before cell rupture to serve as a posthumous mark for a cell that has died by gasdermin-induced pyroptosis, or MLKL-induced necroptosis, for recognition by dendritic cells. This study reveals the spectacular morphology of pyroptosis and identifies a mechanism by which inflammasomes induce pyroptotic cells to construct a de novo alarmin that activates dendritic cells via CLEC9A, which coordinates the transition from innate to adaptive immunity1,2. Pyroptotic cell death results in inflammation. Here the authors find that F-actin-rich structures formed during macrophage pyroptosis persist after cell death to activate dendritic cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


