STING纳米激动剂增强治疗性DNA疫苗的抗肿瘤免疫
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
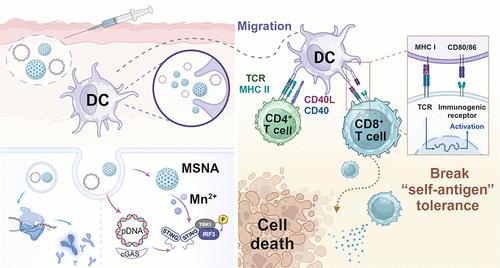
治疗性DNA癌症疫苗可以刺激针对癌症抗原的特异性免疫反应,但往往诱导次优治疗反应。在这里,我们在三种动物模型中证明了锰掺杂二氧化硅纳米颗粒STING激动剂(MSNA)增强质粒DNA疫苗的免疫应答,促进树突状细胞(DC)不同亚群的激活和迁移,并提高抗肿瘤免疫。与α-胎蛋白(AFP)编码的质粒DNA (AFP-DNA)共给药的MSNA引起了比游离AFP-DNA更高的AFP特异性CD8 T细胞反应。用msnna -AFP- dna免疫的动物在表达AFP的肝细胞癌攻击模型中保持无肿瘤。MSNA结合编码人乳头瘤病毒16型癌蛋白E6和E7的DNA质粒,诱导了强效的E7特异性CD8 T细胞反应,阻止了表达E7的实体TC-1肿瘤的生长,促进了表达E7的皮肤移植物的收缩。这些发现共同表明,MSNA的联合施用可以提高靶向癌症特异性抗原的治疗性DNA疫苗的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
STING Nanoagonist Boosts Antitumor Immunity of Therapeutic DNA Vaccines
Therapeutic DNA cancer vaccines can stimulate specific immune responses against cancer antigens but often induce suboptimal therapeutic responses. Here, we demonstrate that a manganese-doped silica nanoparticle STING agonist (MSNA) enhances the immune response of plasmid DNA vaccines, promoting the activation and migration of distinct subsets of dendritic cell (DC) and improving antitumor immunity in three animal models. MSNA coadministered with an α-fetoprotein (AFP) encoded plasmid DNA (AFP-DNA) elicited significantly higher AFP-specific CD8 T cell responses than free AFP-DNA. Animals immunized with MSNA-AFP-DNA remained tumor-free in an AFP expressing hepatocellular carcinoma challenge model. MSNA combined with a DNA plasmid encoding the human papillomavirus type 16 oncoproteins E6 and E7 induced potent E7-specific CD8 T cell responses, preventing the growth of E7-expressing solid TC-1 tumors and promoting the shrinkage of E7-expressing skin grafts. These findings together demonstrate that coadministration of MSNA can improve the efficacy of therapeutic DNA vaccines targeting cancer-specific antigens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


