空肠弯曲杆菌衍生的细胞致死膨胀毒素促进结直肠癌转移
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
各种形式的实体肿瘤都有细胞内细菌,但这些微生物的生理后果尚不清楚。我们发现弯曲杆菌在转移患者的原发性结直肠癌(CRC)病变中显著富集。在肝或肺转移小鼠模型中,空肠弯曲杆菌衍生的细胞致死膨胀毒素(CDT)通过JAK2-STAT3-MMP9信号通路促进结直肠癌转移,这在空肠弯曲杆菌感染的人类结肠组织和CDT治疗的结肠类肿瘤患者中得到证实。cdtB基因缺失(ΔcdtB)或纯化的cdtB蛋白表明基因毒素对空肠梭菌的促转移特性至关重要。在c -jejuni定植的小鼠中,产生cdt的c -jejuni向肠外植入肿瘤的易位增加可能导致这些肿瘤的加速转移。总之,这些发现表明,肿瘤内细菌来源的基因毒素加速肿瘤转移,可能为癌症管理开辟新的诊断和治疗途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
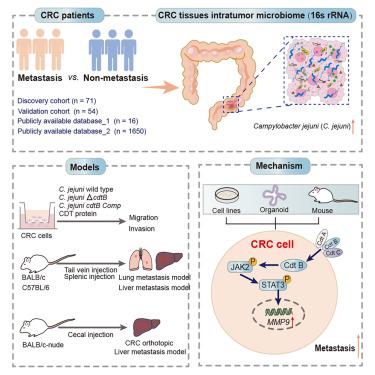
Campylobacter jejuni-derived cytolethal distending toxin promotes colorectal cancer metastasis
Various forms of solid tumors harbor intracellular bacteria, but the physiological consequences of these microorganisms are poorly understood. We show that Campylobacter is significantly enriched in primary colorectal cancer (CRC) lesions from patients with metastasis. Campylobacter jejuni-derived cytolethal distending toxin (CDT) promotes CRC metastasis through JAK2-STAT3-MMP9 signaling in liver or pulmonary metastatic mice models, as confirmed in C. jejuni-infected human colonic tissue and CDT-treated colonic tumoroids from patients. Genetic deletion of cdtB (ΔcdtB) or purified CdtB protein demonstrates that the genotoxin is essential for C. jejuni’s pro-metastatic property. In C.-jejuni-colonized mice, increased translocation of CDT-producing C. jejuni to extraintestinal implanted tumors potentially leads to accelerated metastasis of these tumors. Overall, these findings demonstrate that an intratumor-bacteria-derived genotoxin accelerates tumor metastasis, potentially opening a new diagnostic and therapeutic avenue for cancer management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


