具有无枝晶能力的稳定低温锂金属电池是由电解质与Li+协同溶剂化实现的
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
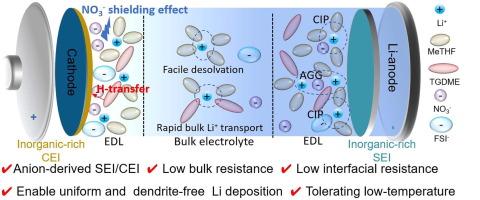
在零下环境中,缓慢的电化学动力学和不稳定的电极/电解质界面阻碍了锂金属电池(lmb)的发展,强调需要先进的电解质来确保恶劣环境下的稳定性。在此,我们提出了一种平衡的“鸡尾酒优化”电解质,通过操纵溶剂化和阴离子。双盐/双溶剂电解质同时实现了低体阻抗和低界面阻抗,同时也表现出更好的Li可逆性和氧化稳定性。定制的溶剂化结构促进阴离子的分解,从而在阴极和锂阳极形成无机富界面,从而实现锂的均匀镀剥离,同时在阴极上保持优异的电压弹性。此外,NO3 -离子优先吸附在阴极表面的内亥姆霍兹平面内,屏蔽了易氧化的非溶剂溶剂分子,这种现象称为“屏蔽效应”,从而抑制了副氧化反应。结果表明,阴离子界面化学有助于无枝晶锂沉积,CE高达99.45%,循环150次后锂||NCM523电池循环稳定,容量保持率高达85%,并且在- 30℃下具有优异的低温放电性能,容量保持率为68.2%。这项工作揭示了在宽温度范围内稳定lmb的令人鼓舞的电解质策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Stable low-temperature lithium metal batteries with dendrite-free ability enabled by electrolytes with cooperative Li+-solvation
In subzero environments, sluggish electrochemical kinetics and unstable electrode/electrolyte interphases hinder progress in lithium metal batteries (LMBs), emphasizing the need for advanced electrolytes to ensure stability in harsh environments. Herein, we proposed a balanced “cocktail optimized” electrolyte by manipulating solvated and anionic species. The dual salt/dual solvent electrolyte simultaneously achieves low bulk impedance and low interfacial impedance, while also demonstrating improved Li reversibility and oxidation stability. The tailored solvation structure encourages the breakdown of anions, leading to the formation of inorganic-rich interphases at both the cathode and Li-anode, which enables a uniform plating-stripping of Li while maintaining exceptional voltage resilience on the cathode. Moreover, NO3– ions preferentially adsorb onto the cathode surface within the inner Helmholtz plane, shielding the easily-oxidized non-solvating solvent molecules, a phenomenon referred to as the “shielding effect”, thus inhibiting side oxidation reactions. Consequently, the anion-derived interface chemistry contributes to the dendrite-free Li deposition with a high CE of 99.45%, a stable cycling of Li||NCM523 battery with 85% capacity retention after 150 cycles, and a superior low-temperature discharge performance at −30 ℃ with a capacity retention of 68.2%. This work sheds light on an encouraging electrolyte strategy for stable LMBs in a wide-temperature range.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


