原子分散ca - n4掺杂石墨烯双分子层中嵌入电子助推器增强CO2对乙醇的电催化作用
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
我们报道了具有单插层碱土金属(AEM)原子的原子分散ca - n4掺杂石墨烯双分子层的发展,标记为CaN4-AEM-CaN4,旨在增强二氧化碳到C1和C2产物的电催化转化。这些催化剂显著提高了CO2还原反应(CO2RR)的活性,并微调了C1和C2途径之间的选择性。基于我们的理论发现,我们提出了一种电子转移机制,其中AEM原子在非吸附中间过程中充当两层的电子供体,在吸附过程中充当电子助推器,促进电子从非吸附层转移到吸附层,从而提高催化性能。AEMs还影响反应途径,通过调节选择性促进更有价值的C2产物的形成。在CaN4-AEM-CaN4体系中,CaN4-Sr-CaN4表现出优异的极限电位(- 0.93 V),表现出优异的co2 -to-乙醇转化的电化学催化活性。本研究强调了CaN4-AEM-CaN4催化剂增强多碳CO2RR的潜力,并为驱动其活性和选择性的电子转移机制提供了关键见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
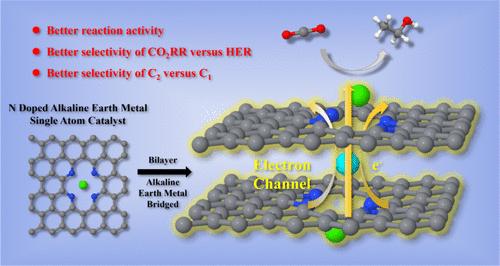
Enhancing Electrocatalysis of CO2 to Ethanol via Intercalated Electron Boosters in an Atomically Dispersed Ca–N4-Doped Graphene Bilayer
We report the development of atomically dispersed Ca–N4-doped graphene bilayers with single intercalated alkaline earth metal (AEM) atoms, denoted as CaN4–AEM–CaN4, designed to enhance the electrocatalytic conversion of CO2 to C1 and C2 products. These catalysts significantly improve the CO2 reduction reaction (CO2RR) activity and fine-tune the selectivity between C1 and C2 pathways. Based on our theoretical findings, we propose an electron transfer mechanism where AEM atoms serve as electron donors across both layers during nonadsorbed intermediate processes and as electron boosters that facilitate electron transfer from the nonadsorbed to the adsorbed layer during adsorption, thereby enhancing catalytic performance. AEMs also influence the reaction pathways, promoting the formation of more valuable C2 products by adjusting selectivity. Among the CaN4–AEM–CaN4 systems, CaN4–Sr–CaN4 stands out with a superior limiting potential (−0.93 V), demonstrating exceptional electrochemical catalytic activity for CO2-to-ethanol conversion. This study underscores the potential of CaN4–AEM–CaN4 catalysts for enhancing multicarbon CO2RR and provides key insights into the electron transfer mechanisms that drive their activity and selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


