乳酸烷基自催化水解的双态反应动力学
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
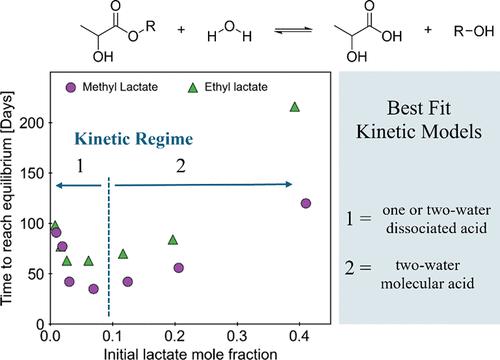
尽管乳酸甲酯和乳酸乙酯在水溶液中可以自发水解,但乳酸烷基酯水解的动力学描述仅限于酸催化条件下。随着反应的进行,生成的乳酸进一步催化酯水解,而反酯化反应的速率也随着酸产物的积累而增加。乳酸水解的反应顺序分为三个动力学阶段:起始/中性水解、自催化水解和平衡。在不同温度(1.5 ~ 40℃)和乳酸甲酯或乳酸乙酯初始浓度(1 ~ 40 mol %)下测定乳酸水解的演变,以量化反应阶段之间的动力学转变。较低的温度导致了明显的诱导期,在此期间可以忽略水解。初始浓度对诱导期长度的影响是非单调的,分为稀释(低于约6摩尔%乳酸)和浓缩(高于约6摩尔%乳酸)两种情况。乳酸浓度越低或越高,诱导期越长,反应越慢。双动力学体系最好地描述了所观察到的水解行为。对于低于10 mol %的乳酸烷基酯水解,源自传统酯水解机制的速率定律有效地模拟了行为,而在较高的乳酸浓度下,必须在速率决定步骤中加入额外的水分子以适当地捕获水解行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual-Regime Reaction Kinetics of the Autocatalytic Hydrolyses of Aqueous Alkyl Lactates
Kinetic description of the hydrolysis of alkyl lactates has been limited to acid-catalyzed conditions despite the spontaneous hydrolysis of methyl lactate and ethyl lactate in aqueous solution. As the reaction progresses, generated lactic acid further catalyzes ester hydrolysis, while the rate of the reverse esterification reaction also increases with the accumulation of acid product. The reaction sequence of lactate hydrolysis is described in three kinetic stages: initiation/neutral hydrolysis, autocatalytic hydrolysis, and equilibrium. The evolution of lactate hydrolysis was measured for varying temperatures (1.5 to 40 °C) and initial concentrations of methyl or ethyl lactate (1 to 40 mol %) to quantify the kinetic transitions between reaction stages. Lower temperatures resulted in a distinct induction period where negligible hydrolysis was observed. The effect of initial concentration on the length of the induction period was nonmonotonic and was divided into dilute (below about 6 mol % lactate) and concentrated (above about 6 mol %) regimes. Solutions of either lower or higher lactate concentration corresponded to longer induction periods and slower reactions. A dual kinetic regime best describes the observed hydrolysis behavior. For hydrolysis of alkyl lactates below 10 mol %, a rate law derived from the conventional ester hydrolysis mechanism effectively modeled behavior, while at higher lactate concentrations, an additional water molecule must be included in the rate-determining step to appropriately capture the hydrolysis behavior.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


