脂肪变性肝病和过量酒精摄入患者酒精摄入量的定量分析
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
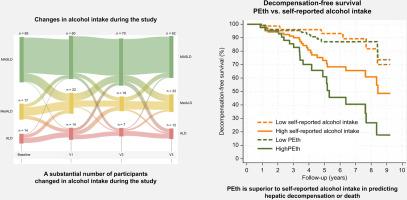
背景,目的量化酒精摄入量对于脂肪变性肝病(SLD)患者的亚分类以及解释酒精相关肝病(ALD)、代谢性和酒精相关肝病(MetALD)的临床试验至关重要。然而,自我报告的酒精摄入量的准确性被认为是不精确的。我们比较了自我报告的酒精摄入量与基于血液的酒精摄入量生物标志物:磷脂酰乙醇(PEth)和碳水化合物缺乏转铁蛋白(CDT)的诊断和预后效用。方法:我们研究了192名来自MetALD和ALD两项随机对照试验的参与者,所有参与者目前或曾经过量饮酒(≥24/36[♀/♂]克每天至少1年),并活检证实有肝脏疾病。我们在四个时间点评估了自我报告的酒精摄入量、PEth和CDT。我们通过电子病历手工收集肝失代偿和死亡的随访数据。结果大多数参与者为男性(n = 161, 84%),平均年龄59岁(SD 9), 73名参与者在纳入前报告了1周的禁欲;其余的人平均每天摄入43克酒精。中位PEth为0.5 μmol/L (IQR: 0.0 ~ 1.3), %CDT = 1.9 (IQR: 1.6 ~ 2.3)。32例患者报告至少禁欲6个月;27个(84%)经PEth 0.05 μmol/L确证。自我报告的酒精摄入量与PEth有良好的相关性(r = 0.617),与CDT有中度相关性(r = 0.316)。自我报告的酒精摄入量、PEth和CDT均可预测肝功能失代偿和死亡。然而,PEth的预测最高,超过了自我报告的酒精摄入量(Harrel 's C, PEth = 0.80 vs.自我报告= 0.68,p = 0.026)。结论自我报告戒断在临床试验中是可靠的。然而,PEth在预测MetALD和ALD患者肝功能失代偿和死亡方面具有优势。影响和意义准确量化酒精摄入量对脂肪肝患者的临床表型和临床试验设计至关重要。该研究发现自我报告的戒断是可靠的,但在临床试验中,磷脂酰乙醇是肝失代偿和死亡的更准确的预后生物标志物。研究结果可能为未来脂肪变性肝病患者试验的设计提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quantification of alcohol intake in patients with steatotic liver disease and excessive alcohol intake
Background & Aims
Quantifying alcohol intake is crucial for subclassifying participants with steatotic liver disease (SLD) and interpreting clinical trials of alcohol-related liver disease (ALD) and metabolic and alcohol-related liver disease (MetALD). However, the accuracy of self-reported alcohol intake is considered imprecise. We compared the diagnostic and prognostic utility of self-reported alcohol intake with blood-based biomarkers of alcohol intake: phosphatidylethanol (PEth) and carbohydrate-deficient transferrin (CDT).
Methods
We studied 192 participants from two randomized controlled trials on MetALD and ALD, all with current or former excessive alcohol intake (≥24/36 [♀/♂] grams daily for at least 1 year) and biopsy-proven liver disease. We assessed self-reported alcohol intake, PEth, and CDT at four time points. We collected follow-up data on hepatic decompensation and death manually through electronic medical records.
Results
Most participants were male (n = 161, 84%) with a mean age of 59 (SD 9) years and 73 participants reported 1-week abstinence before inclusion; the remaining reported a median alcohol intake of 43 g/day. Median PEth was 0.5 μmol/L (IQR: 0.0–1.3) and %CDT = 1.9 (IQR: 1.6–2.3). Of 32 patients reporting at least 6 months of abstinence; 27 (84%) was confirmed by PEth <0.05 μmol/L. Self-reported alcohol intake correlated well with PEth (r = 0.617) and moderately with CDT (r = 0.316). Self-reported alcohol intake, PEth, and CDT all predicted hepatic decompensation and death. However, PEth showed the highest prediction, surpassing self-reported alcohol intake (Harrel’s C, PEth = 0.80 vs. self-reported = 0.68, p = 0.026).
Conclusions
Self-reported abstinence can be considered reliable in clinical trials. However, PEth is superior in predicting hepatic decompensation and death in patients with MetALD and ALD.
Impact and implications
An accurate quantification of alcohol intake is crucial in the clinical phenotyping of patients with steatotic liver disease and when designing clinical trials. This study found self-reported abstinence to be reliable but phosphatidylethanol was a more accurate prognostic biomarker of hepatic decompensation and death in a clinical trial setting. Findings may inform the design of future trials in patients with steatotic liver disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


