苯丙嗪和替莫唑胺在胶质母细胞瘤中的协同抗肿瘤作用:机制和分子靶向
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
胶质母细胞瘤(GBM)是最具侵袭性的脑癌之一,由于其复杂的生物学和对常规治疗的耐药性,提出了重大的治疗挑战,需要开发新的、低毒的、有效的治疗方法。本研究探讨了phenlorizin(一种天然存在的二氢查尔酮)作为单独药物和与替莫唑胺(TMZ)联合使用的抗肿瘤潜力,替莫唑胺是GBM的标准化疗药物。研究发现,苯连菌素对体外细胞活力和迁移有显著抑制作用,并与TMZ联用有协同作用。综合分析,包括蛋白-蛋白相互作用网络构建、富集分析以及与AKT1的分子对接,发现PI3K/AKT/mTOR信号通路是根霉素靶向胶质母细胞瘤细胞存活和增殖的关键介质。88个交叉靶点的通路富集分析进一步强调了该通路在苯连菌素活性中的作用。Western blot验证证实,phlorizin抑制PI3K/AKT/mTOR通路关键蛋白的表达,为其抗肿瘤作用提供了机制基础。这些发现表明,phlorizin,特别是与TMZ联合使用,通过靶向对癌细胞存活和增殖至关重要的分子通路,具有作为胶质母细胞瘤治疗策略的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
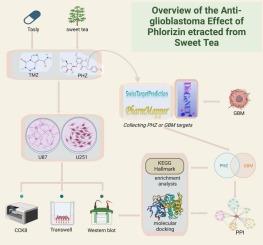
Synergistic antitumor effects of Phlorizin and Temozolomide in glioblastoma: Mechanistic insights and molecular targeting
Glioblastoma (GBM), one of the most aggressive brain cancers, presents significant treatment challenges due to its complex biology and resistance to conventional therapies, necessitating the development of new, low-toxicity, and effective treatments. This study explores the antitumor potential of phlorizin, a naturally occurring dihydrochalcone, as a standalone agent and in combination with temozolomide (TMZ), the standard chemotherapeutic for GBM. Phlorizin was found to significantly inhibit cell viability and migration in vitro, with synergistic effects observed when combined with TMZ. Comprehensive analyses, including protein-protein interaction network construction, enrichment analysis, and molecular docking with AKT1, identified the PI3K/AKT/mTOR signaling pathway as a critical mediator of glioblastoma cell survival and proliferation targeted by phlorizin. Pathway enrichment analysis of 88 intersection targets further highlighted this pathway's role in phlorizin's activity. Western blot validation confirmed that phlorizin inhibits the expression of key proteins within the PI3K/AKT/mTOR pathway, providing a mechanistic basis for its antitumor effects. These findings suggest that phlorizin, particularly in combination with TMZ, holds significant potential as a therapeutic strategy for glioblastoma by targeting molecular pathways critical for cancer cell survival and proliferation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


