通过hyperperforin靶向plat - trpv3通路,诱导非规范促进脂肪产热,作为一种有效的抗肥胖策略
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
促进脂肪产热被认为是一种很有前途的肥胖治疗干预措施。然而,针对典型的产热调节途径,特别是β3-肾上腺素能受体(β3-AR)依赖机制开发抗肥胖药物的努力由于β3-AR激动剂的脱靶作用而失败,加剧了心血管疾病的风险。hyperperin (HPF)是一种从传统草药圣约翰草中提取的天然化合物,它与二氢脂酰胺s-乙酰转移酶(Dihydrolipoamide s-acetyltransferase, Dlat)结合,通过促进脂肪产热发挥有效的抗肥胖作用。目的观察HPF的口服药效和药代动力学特征,探讨dfat调节HPF介导的脂肪产热的具体机制。方法为了评估口服高脂多糖的体内抗肥胖效果,采用高脂饮食(HFD)喂养的Dlat杂合敲除(Dlat+/-)小鼠和野生型(WT)小鼠进行了代谢笼、核磁共振分析和红外成像的验证过程。采用Sprague Dawley大鼠测定HPF的药动学参数。采用海马实验、JC-1染色、qPCR和免疫印迹法评价HPF和Dlat在体外的细胞产热作用。结果我们的研究发现了一个非典型的产热途径,涉及到Dlat、瞬时受体电位香草酸样蛋白3 (Trpv3,一种钙通道)和AMPK。Dlat与Trpv3相互作用激活它,导致细胞内钙(Ca2+)的增加和Camkkβ的激活。然后Camkkβ刺激AMPK,导致Ucp1表达升高并启动脂肪产热。HPF通过增强不依赖于β3-AR的plat - trpv3相互作用促进脂肪组织产热,对心脏的副作用最小。值得注意的是,HPF在Dlat+/-小鼠中的产热作用降低。此外,HPF具有良好的口服生物利用度、相对较长的半衰期和在脂肪组织中的广泛分布。综上所述,我们的研究表明,HPF是一种促进脂肪产热的新机制,具有有效和安全的抗肥胖功效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
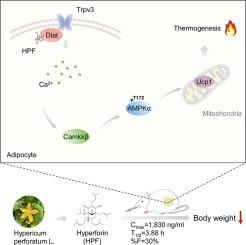
Targeting Dlat-Trpv3 pathway by hyperforin elicits non-canonical promotion of adipose thermogenesis as an effective anti-obesity strategy
Introduction
Promoting adipose thermogenesis is considered as a promising therapeutic intervention in obesity. However, endeavors to develop anti-obesity medications by targeting the canonical thermogenesis regulatory pathway, particularly β3-adrenergic receptor (β3-AR)-dependent mechanism, have failed due to the off-target effects of β3-AR agonists, exacerbating the risk of cardiovascular disease. Hyperforin (HPF), a natural compound extracted from the traditional herbal St. John’s Wort, binds to Dihydrolipoamide s-acetyltransferase (Dlat) and exerts effective anti-obesity properties through promoting adipose thermogenesis.Objectives
The objective of this study was to investigate the oral efficacy and pharmacokinetics profile of HPF, and explore the detailed mechanism by which Dlat modulates HPF-mediated adipose thermogenesis.Methods
To assess the anti-obesity efficacy of orally administered HPF in vivo, Dlat heterozygous knockout (Dlat+/-) mice and wild-type (WT) mice, both fed a high-fat diet (HFD), underwent a validation process that involved the use of metabolic cages, NMR analysis, and infrared imaging. Sprague Dawley rats were employed to determine the pharmacokinetic parameters of HPF. Seahorse assays, JC-1 staining, qPCR, and immunoblotting were performed to evaluate cellular thermogenic efficacy of HPF and Dlat in vitro.Results
Our study uncovered a non-canonical thermogenesis pathway involving Dlat, transient receptor potential vanilloid 3 (Trpv3, a calcium channel) and AMPK. Dlat interacted with Trpv3 to activate it, resulting in an increase in intracellular calcium (Ca2+) and the activation of Camkkβ. Camkkβ then stimulated AMPK, leading to elevated Ucp1 expression and initiating adipose thermogenesis. HPF promoted thermogenesis in adipose tissues through enhancing the Dlat-Trpv3 interaction independently of β3AR, causing minimal cardiac side effects. Notably, HPF’s thermogenic effects were reduced in Dlat+/- mice. Moreover, HPF exerted favorable oral bioavailability, a relatively long half-life, and extensive distribution within adipose tissues.Conclusion
In summary, our study demonstrates that HPF targets a novel mechanism for promoting adipose thermogenesis and exhibits potent and safe anti-obesity efficacy.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


