可见光诱导的亚砜鎓化物与叠氮化物的级联环合成2-三氟甲基吲哚
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了一种通过可见光促进的亚砜鎓化物与叠氮化物的分子间环化合成2-三氟甲基吲哚的有效方案,而不需要外部光催化剂、过渡金属或碱。2-三氟甲基吲哚的形成涉及一个有趣的级联过程,包括叠氮化物重排、分子间亲核加成和关键中间体的可见光促进环化。该工艺具有效率高、条件温和、底物相容性好、区域选择性好等特点。通过放大反应和对合成吲哚的进一步修饰,证明了这种方法的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
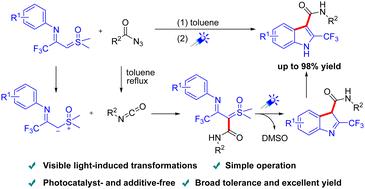
Visible light-induced cascade annulation of sulfoxonium ylides with azides for the synthesis of 2-trifluoromethyl indoles†
An efficient protocol was reported for the synthesis of 2-trifluoromethyl indoles through visible-light-promoted intermolecular cyclization of sulfoxonium ylides with azides, without the need for external photocatalysts, transition metals, or bases. The formation of 2-trifluoromethyl indoles involves an intriguing cascade process including azide rearrangement, intermolecular nucleophilic addition, and visible-light-promoted cyclization of a key intermediate. The protocol features high efficiency, mild conditions, excellent substrate compatibility and good regioselectivity. The practicality of this approach is demonstrated through scale-up reactions and further modifications of the synthesized indoles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


