分子复杂性的快速途径:钯催化的六重多米诺过程获得多环框架**
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们提出了一种迄今为止尚未探索的有效策略,用于快速构建具有两个相邻四元中心的结构约束和有趣的多环框架。值得注意的是,这可以通过钯催化简单的1,2-双(2-溴芳基)乙炔和1,2-二乙炔的六倍多米诺交叉环实现。值得注意的是,这种方法证明了c2对称和非对称多环产物的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
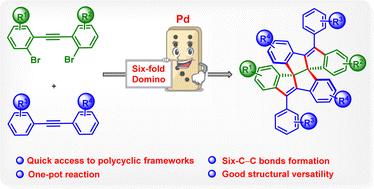
A rapid pathway to molecular complexity: a palladium-catalyzed six-fold domino process to access polycyclic frameworks†
Herein, we present a hitherto unexplored efficient strategy for rapidly constructing structurally constrained and intriguing polycyclic frameworks with two adjacent quaternary centers. Remarkably, this becomes possible through palladium-catalyzed six-fold domino crossover annulations of simple 1,2-bis(2-bromoaryl)ethynes and 1,2-diarylethynes. Notably, this approach demonstrates the synthesis of both C2-symmetric and unsymmetric polycyclic products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


