lini0.83 mn0.05 co0.120 o2在水浆中电化学性能劣化机理及缓解策略
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
将高镍层状氧化物阴极与水浆电极制备路线相结合,有可能同时满足锂离子电池高能量密度和低成本生产的需求。然而,空气和液态水的双重暴露以及水浆电极加工过程中的加热处理对高镍层状氧化物电极的影响尚不清楚。在本研究中,我们系统地研究了lini0.83 mn0.05 co0.120 o2 (NMC83)在水浆处理过程中的结构演变和电化学行为。结果表明,NMC83表面附近的晶体结构部分重构为岩盐相和层状相的混合物,导致NMC83电极的速率性能劣化。这部分表面重构层经过循环后完全转化为纯岩盐相,并伴有O2的释放、Ni的浸出、电解质的催化分解,形成较厚的阴极电解质间相层。电解质的副产物和溶解的Ni可以穿梭到Li金属侧,引起串扰效应,导致Li表面上厚而不稳定的固体电解质界面层。这些因素的结合严重破坏了从水泥浆中获得的NMC83电极的循环稳定性。采用分子自组装技术的缓释策略可增强水处理后的NMC83的表面稳定性。我们的发现为定制超高镍层状氧化物和其他水敏阴极材料的环境稳定性和水浆可加工性提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
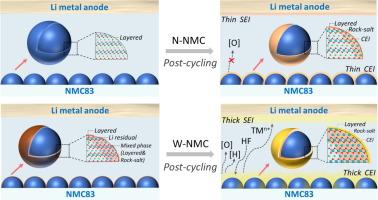
The electrochemical performance deterioration mechanism of LiNi0.83Mn0.05Co0.12O2 in aqueous slurry and a mitigation strategy
Integrating high-nickel layered oxide cathodes with aqueous slurry electrode preparation routes holds the potential to simultaneously meet the demands for high energy density and low-cost production of lithium-ion batteries. However, the influence of dual exposure to air and liquid water as well as the heating treatment during aqueous slurry electrode processing on the high-nickel layered oxide electrode is yet to be understood. In this study, we systematically investigate the structural evolution and electrochemical behaviors when LiNi0.83Mn0.05Co0.12O2 (NMC83) is subjected to aqueous slurry processing. It was observed that the crystal structure near the surface of NMC83 is partially reconstructed to contain a mixture of rock-salt and layered phases when exposed to water, leading to the deteriorated rate capability of the NMC83 electrodes. This partial surface reconstruction layer completely converts into a pure rock-salt phase upon cycling, accompanied by the release of O2, Ni leaching, catalyzed decomposition of the electrolyte, and the formation of a thick cathode electrolyte interphase layer. The byproducts of the electrolyte and dissolved Ni could shuttle to the Li metal side, causing a crosstalk effect that results in a thick and unstable solid electrolyte interphase layer on the Li surface. These in combination severely undermined the cycling stability of the NMC83 electrodes obtained from the aqueous slurry. A mitigation strategy using molecular self-assembly technique was demonstrated to enhance the surface stability of water-treated NMC83. Our findings offer new insights for tailoring ambient environment stability and aqueous slurry processability for ultra-high nickel layered oxide and other water-sensitive cathode materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


