间充质干细胞工程类骨器官方案
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨类器官是研究骨发育及相关疾病的有力工具。然而,现有方法的简化设计在一定程度上限制了它们的应用潜力,因为这些方法产生的单组织类器官无法复制骨微结构或实现有效的矿化。为了解决这一问题,我们提出了一种利用骨髓间充质干细胞(BMSCs)生成矿化骨结构的三维(3D)构建策略。通过将骨髓间充质干细胞与水凝胶混合制成骨基质模拟生物墨水,并采用基于投影的光固化3D打印技术,我们构建了3D打印结构,然后将其植入裸鼠皮下,远离天然骨微环境。即使没有外部刺激,这些植入物也会自发形成矿化骨域。随着长期培养,这些结构逐渐成熟为完全分化的骨组织,完成矿化和血管化。这种体内骨类器官模型为研究骨骼发育、探索先天性疾病、测试药物和开发治疗应用提供了一个新的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
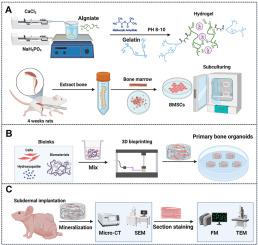
Protocol for engineering bone organoids from mesenchymal stem cells
Bone organoids are emerging as powerful tools for studying bone development and related diseases. However, the simplified design of current methods somewhat limits their application potential, as these methods produce single-tissue organoids that fail to replicate the bone microarchitecture or achieve effective mineralization. To address this issue, we propose a three-dimensional (3D) construction strategy for generating mineralized bone structures using bone marrow-derived mesenchymal stem cells (BMSCs). By mixing BMSCs with hydrogel to create a bone matrix-mimicking bioink and employing projection-based light-curing 3D printing technology, we constructed 3D-printed structures, which were then implanted subcutaneously into nude mice, away from the native bone microenvironment. Even without external stimulation, these implants spontaneously formed mineralized bone domains. With long-term culture, these structures gradually matured into fully differentiated bone tissue, completing both mineralization and vascularization. This in vivo bone organoid model offers a novel platform for studying bone development, exploring congenital diseases, testing drugs, and developing therapeutic applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


