成人先天性心脏病无导线起搏器与皮下植入式心律转复除颤器联合应用的可行性试验1例报告
Q4 Medicine
引用次数: 0
摘要
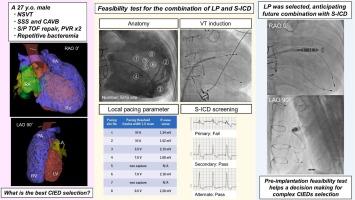
新的心脏植入式电子装置(cied),如无导线起搏器和皮下植入式心律转复除颤器(S-ICDs),正在用于成人先天性心脏病患者。由于独特的心脏结构带来的技术挑战,cied的选择通常需要仔细考虑。一位27岁的男性在接受外科法洛四联症(TOF)修复后出现了非持续性室性心动过速、病态窦综合征和完全性房室传导阻滞。他有复发性菌血症史。在考虑了感染风险后,我们讨论了使用无导线起搏器和S-ICD作为非经静脉CIED的组合,并在植入前测试后决定选择合适的CIED。电生理研究未发现室性心动过速。虽然他当时不需要ICD,但TOF修复后的患者在以后的生活中发生室性心动过速的风险很高。我们测量了局部起搏阈值和r波振幅,并对起搏qrs进行了S-ICD筛选。最后,我们安全地植入了一个无铅起搏器,如果需要,可以选择添加S-ICD。在成人先天性心脏病患者中,植入前测试可以帮助将来决定是否将无导联起搏器与s - icd联合使用。学习目的成人先天性心脏病患者心脏植入式电子装置(CIED)的合理选择需要慎重考虑。无导联起搏器(LP)与皮下植入式心律转复除颤器结合的植入前可行性测试有助于在独特的心脏结构中选择CIED和安全的LP植入过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A case report of pre-implantation feasibility test for combining leadless pacemaker and subcutaneous implantable cardioverter-defibrillator in adult congenital heart disease
New cardiac implantable electronic devices (CIEDs), such as leadless pacemakers and subcutaneous implantable cardioverter defibrillators (S-ICDs), are being used in patients with adult congenital heart disease. The selection of CIEDs often requires careful consideration due to technical challenges posed by a unique heart structure. A 27-year-old man following a surgical tetralogy of Fallot (TOF) repair developed non-sustained ventricular tachycardia, sick sinus syndrome, and complete atrioventricular block. He had a history of recurrent bacteremia. We discussed the use of a combination of leadless pacemaker and S-ICD as a non-transvenous CIED after considering the infection risk and decided to select the appropriate CIED after a pre-implantation test. Ventricular tachycardia was not induced in the electrophysiological study. Although he did not need an ICD at that point, patients after TOF repair are at a high risk for ventricular tachycardia later in life. We measured the local pacing threshold and R-wave amplitude and performed an S-ICD screening for paced-QRS. Finally, we implanted a leadless pacemaker safely with the option to add an S-ICD if needed. A pre-implantation test could help future decisions regarding combinations of leadless pacemakers with S-ICDs in patients with adult congenital heart disease.
Learning objectives
The appropriate selection of a cardiac implantable electronic device (CIED) in patients with adult congenital heart disease requires careful consideration. The pre-implantation feasibility test for combining a leadless pacemaker (LP) and a subcutaneous implantable cardioverter defibrillator aided decision-making in CIED selection and safe LP implantation procedure in the unique heart structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cardiology Cases
Medicine-Cardiology and Cardiovascular Medicine
CiteScore
0.90
自引率
0.00%
发文量
177
审稿时长
59 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


