光催化析氢用三聚氰胺基聚合物的水热合成
IF 5.8
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
太阳能光催化水裂解析氢已被公认为一种很有前途的制氢技术,开发高效、廉价、实用的新型光催化剂是实现该技术的关键。与半导体光催化剂相比,聚合物光催化剂因其结构多样性高和带隙可调而得到广泛应用。聚合光催化剂的合成过程一般比较复杂,反应条件也比较苛刻(如无氧、使用催化剂等)。本文以三聚氰胺(MA)和对邻苯二醛(PPA)为前驱体,在无添加剂的空气气氛下,采用简单一步水热法合成了三聚氰胺基聚合物(MP-1和MP-2)。MP-1和MP-2在Pt作为助催化剂和TEOA作为牺牲孔清除剂存在下,表现出光催化氢的析出。讨论了不同聚合物结构对光催化析氢的影响。MP-1的析氢速率为1784.2 umol·h−1·g−1,明显高于MP-2的1139.8 umol·h−1·g−1。通过电化学测量和PL研究了MP-1和MP-2的光诱导载流子的分离和迁移。认为MP-1的亚胺(c = N -)结构具有良好的共轭体系,与MP-2的动物结构(- N - c - N -)相比,在光激发下可以产生更多的光诱导电子-空穴对,并且电荷迁移也更容易。该研究有望为利用太阳能光催化制备“绿色氢”而不是合成易降解聚合物光催化剂做出贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
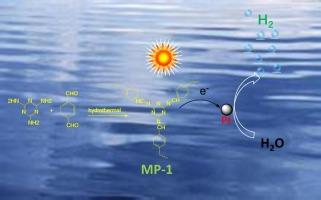
Facile hydrothermal synthesis of melamine-based polymers for photocatalytic hydrogen evolution
Solar photocatalytic hydrogen evolution from water splitting has been recognized as a promising hydrogen production technology, the development of efficient, cheap, and practical new photocatalysts is the key to realizing this technology. Compared with semiconductor photocatalysts, polymeric photocatalysts have emerged due to their high structural diversity and adjustable band gaps. The synthesis process of the polymeric photocatalysts is generally complicated, and the reaction conditions are also harsh (such as oxygen free, using catalyst). In this work, melamine-based polymers (MP-1 and MP-2) were synthesized by a simple one-step hydrothermal method using melamine (MA) and p-phthalaldehyde (PPA) as precursors under air atmosphere without any additives. MP-1 and MP-2 display photocatalytic H2 evolution from water splitting in the presence of Pt as a co-catalyst and TEOA as a sacrificial hole scavenger. The effect of different structure of polymers on photocatalytic H2 evolution was discussed. The hydrogen evolution rate of MP-1 is 1784.2 umol·h−1·g−1, distinctly higher than that of MP-2 (1139.8 umol·h−1·g−1). The separation and migration of photoinduced carriers for MP-1 and MP-2 were investigated by electrochemical measurements and PL. It is thought that the imine (–C = N–) structure of MP-1 has a good conjugated system, which could generate more photoinduced electron-hole pairs under light excitation, and the charge migration is also more facile, compared with the aminal structure (–N–C–N–) of MP-2. This study is expected to contribute toward the development of “green hydrogen” using solar photocatalysis over synthetically facile polymeric photocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Saudi Chemical Society
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
8.90
自引率
1.80%
发文量
120
审稿时长
38 days
期刊介绍:
Journal of Saudi Chemical Society is an English language, peer-reviewed scholarly publication in the area of chemistry. Journal of Saudi Chemical Society publishes original papers, reviews and short reports on, but not limited to:
•Inorganic chemistry
•Physical chemistry
•Organic chemistry
•Analytical chemistry
Journal of Saudi Chemical Society is the official publication of the Saudi Chemical Society and is published by King Saud University in collaboration with Elsevier and is edited by an international group of eminent researchers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


