破骨细胞对纳米钛表面生长成骨细胞长链非编码rna表观遗传调控的影响
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-11-29
DOI:10.1016/j.bioadv.2024.214128
引用次数: 0
摘要
钛(Ti)种植体骨整合受表观遗传机制影响的骨细胞间的串扰调控,包括长链非编码rna (lncRNAs)的调控。纳米形貌钛(Ti Nano)通过表观遗传机制诱导被破骨细胞抑制的成骨细胞分化。因此,我们假设破骨细胞影响钛纳米培养成骨细胞中lncRNA的表达。成骨细胞分别在Ti Nano和Ti Control上生长,然后与破骨细胞共培养48小时。使用RNAseq,我们在受Ti两种表面调控的成骨细胞中鉴定出252种可调节的lncrna,但主要存在于Ti纳米培养的成骨细胞中。在钛纳米培养的破骨细胞中,Kcnq1ot1与Alpl、Bglap、Bmp8a、Col1a1、Vim mrna呈负相关。下拉结果表明,Bglap mRNA是kcnqot1的直接靶点,在Ti纳米培养的成骨细胞中具有增强的物理相互作用,并且比Ti对照具有更大的破骨细胞抑制作用。kcnqot1沉默后,骨标志物mRNA和蛋白水平表达下调,提示其在成骨细胞分化中起关键作用。这些结果表明,纳米结构的钛表面至少在一定程度上通过调控成骨细胞中lncRNA的表达来调节成骨细胞与破骨细胞的串扰。我们发现lncRNA kcnqot1直接与Bglap mRNA相互作用,并且在钛纳米培养的成骨细胞中,这种相互作用被纳米形貌增强,被破骨细胞减弱,且强度更大。这些发现证实了与纳米形貌高成骨潜能相关的分子机制,并可能支持牙齿和骨骼假体的骨整合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
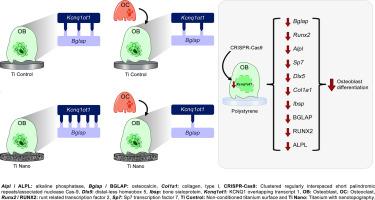
The effect of osteoclasts on epigenetic regulation by long non-coding RNAs in osteoblasts grown on titanium with nanotopography
Titanium (Ti) implant osseointegration is regulated by the crosstalk among bone cells that are affected by epigenetic machinery, including the regulation of long non-coding RNAs (lncRNAs). Nanotopography Ti (Ti Nano) induces the differentiation of osteoblasts that are inhibited by osteoclasts through epigenetic mechanisms. Thus, we hypothesize that osteoclasts affect lncRNA expression in Ti Nano-cultivated osteoblasts. Osteoblasts were grown on Ti Nano and Ti Control that were then co-cultured with osteoclasts for 48 h. Using RNAseq, we identified 252 modulated lncRNAs in osteoblasts regulated by both surfaces of Ti, but mainly in Ti Nano-cultivated osteoblasts. A negative correlation was observed between Kcnq1ot1 and the mRNAs of Alpl, Bglap, Bmp8a, Col1a1, and Vim in Ti Nano-cultivated osteoblasts with osteoclasts. The pull-down indicated that Bglap mRNA is a direct target of Kcnq1ot1, with enhanced physical interaction in Ti Nano-cultivated osteoblasts, and greater osteoclast inhibition than the Ti Control. The bone marker expression at the levels of mRNA and protein were downregulated by the Kcnq1ot1 silencing, indicating its pivotal role in osteoblast differentiation. These results showed that nanostructured Ti surface modulates the osteoblast–osteoclast crosstalk, at least in part, through the regulation of lncRNA expression in osteoblasts. We demonstrate that the lncRNA Kcnq1ot1 directly interacts with Bglap mRNA, and this interaction is enhanced by nanotopography and reduced by osteoclasts with greater intensity in Ti Nano-cultivated osteoblasts. These findings confirm the molecular mechanisms associated with the high osteogenic potential of nanotopography and can potentially support osteointegration of dental and skeletal prostheses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


