五味子内酯A和B:两种五味子内酯,骨架为14,15-seco-15,17- cycloolanciforartane。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究从五味子(Schisandra incarnata Stapf)茎中分离得到了两个先前未被描述的具有前所未有的5/5/6/5/7/5融合六环骨架的五味子三萜(snt) A(1)和B(2),以及一个先前未被描述的(3)和一个已知的(4)类似物。通过综合光谱分析、x射线晶体学和电子圆二色性计算确定了它们的绝对构型。化合物1和2代表了第一类14,15-seco-15,17-环ancianciartane -type snt,其中含有异常的-C14-C16-C13-C17-C15连锁。假设了化合物1和2的生物发生途径。化合物1和2表现出较强的α-葡萄糖苷酶抑制活性,IC50值分别为133.1和165.3 μM,比阳性对照阿卡波糖(IC50 = 232.8 μM)的抑制活性更强。化合物4对cona诱导的T细胞和lps诱导的B细胞增殖具有中等的体外免疫抑制作用,IC50值分别为35.3±0.9 μM和24.9±0.6 μM。化合物1-4对三种人类癌细胞系的细胞毒性也进行了测试,未观察到明显的细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
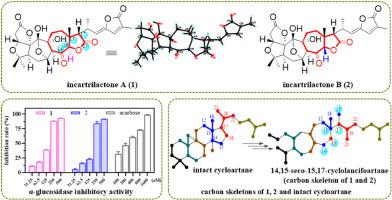
Incartrilactones A and B: Two schinortriterpenoids with a 14,15-seco-15,17-cyclolancifoartane skeleton from Schisandra incarnata
In this study, incartrilactones A (1) and B (2), two previously undescribed schinortriterpenoids (SNTs) possessing an unprecedented 5/5/6/5/7/5-fused hexacyclic skeleton, together with one previously undescribed (3) and one known (4) analogues, were isolated from the stems of Schisandra incarnata Stapf. Their structures with absolute configurations were determined by comprehensive spectroscopic analysis, X-ray crystallography and electronic circular dichroism calculation. Compounds 1 and 2 represent the first class of 14,15-seco-15,17-cyclolancifoartane-type SNTs containing the unusual linkage of ‒C14‒C16‒C13‒C17‒C15. The hypothetical biogenetic pathway of compounds 1 and 2 was postulated. Compounds 1 and 2 exhibited potent α-glucosidase inhibitory activity with IC50 values of 133.1 and 165.3 μM, representing that they were more active than the positive control, acarbose (IC50 = 232.8 μM). Compound 4 showed moderate in vitro immunosuppressive effect against ConA-induced T cell and LPS-induced B cell proliferation, with IC50 values of 35.3 ± 0.9 μM and 24.9 ± 0.6 μM, respectively. The cytotoxicity of compounds 1−4 against three human cancer cell lines was also tested, with no obvious cytotoxicity being observed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


