计算驱动的类似nrp的羧酸还原酶的重新设计提高了活性和选择性。
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
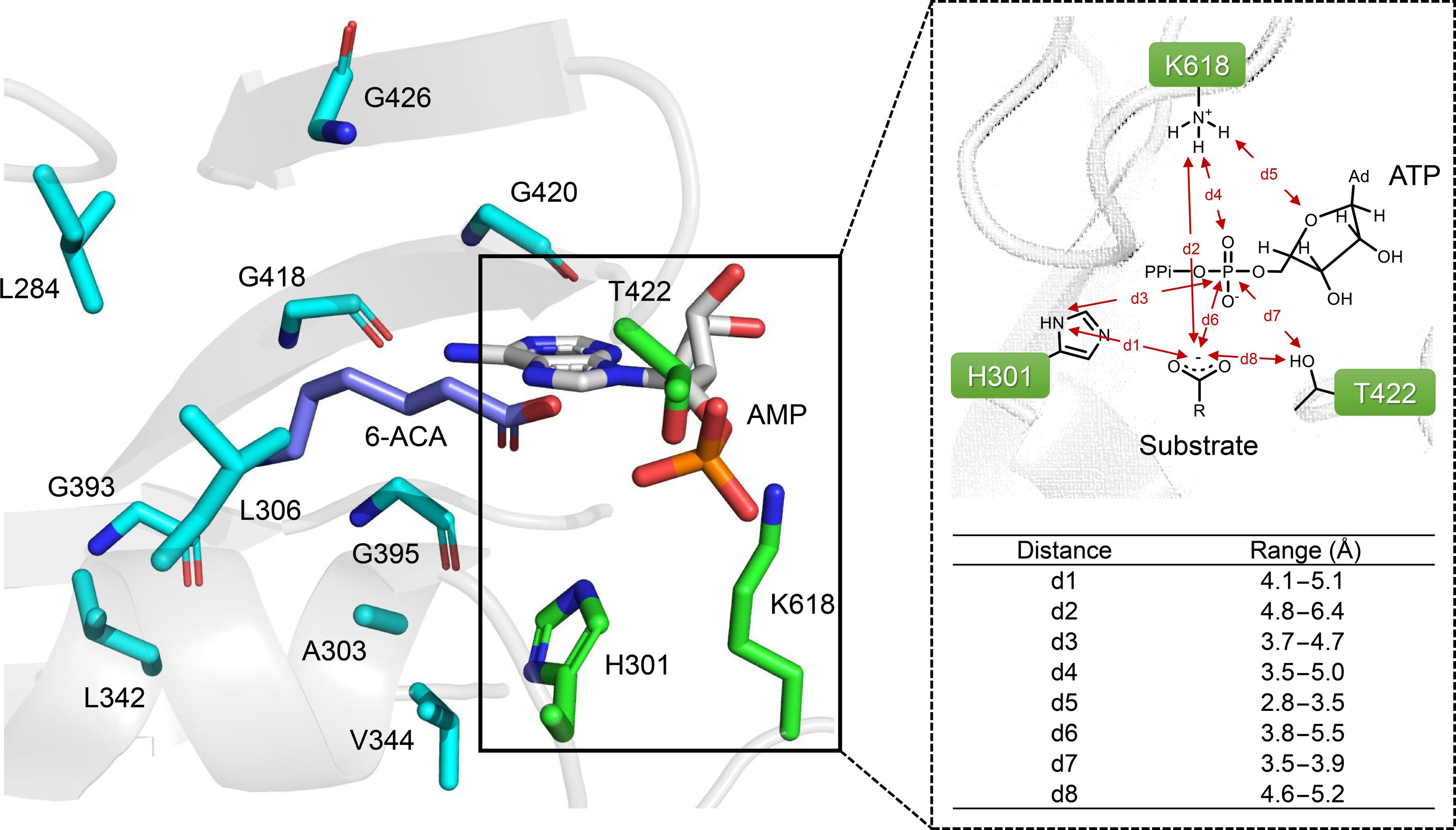
工程非核糖体肽合成酶(NRPSs)由于其模块化特性和对催化机制的有限理解,一直是合成生物学中的“圣杯”。在这里,我们报道了一种基于近似机制的几何标准和Rosetta能量评分的计算重新设计了模型nrpps类酶羧酸还原酶(CARs)的“看门人”腺苷化结构域。值得注意的是,MabCAR3突变体ACA-1和ACA-4对6-氨基己酸的催化效率(kcat/KM)显著提高,达到101倍。此外,G418K对己二酸的底物特异性比6-氨基己二酸增强了86倍。我们的工作不仅为尼龙单体生物合成提供了有前途的生物催化剂,而且为高效的NRPSs工程提供了策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Computation-driven redesign of an NRPS-like carboxylic acid reductase improves activity and selectivity
Engineering nonribosomal peptide synthetases (NRPSs) has been a “holy grail” in synthetic biology due to their modular nature and limited understanding of catalytic mechanisms. Here, we reported a computational redesign of the “gate-keeper” adenylation domain of the model NRPS-like enzyme carboxylic acid reductases (CARs) by using approximate mechanism-based geometric criteria and the Rosetta energy score. Notably, MabCAR3 mutants ACA-1 and ACA-4 displayed a remarkable improvement in catalytic efficiency (kcat/KM) for 6-aminocaproic acid, up to 101-fold. Furthermore, G418K exhibited an 86-fold enhancement in substrate specificity for adipic acid compared to 6-aminocaproic acid. Our work provides not only promising biocatalysts for nylon monomer biosynthesis but also a strategy for efficient NRPSs engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


