假单胞菌松脂醇羟化酶的生化和结构研究。
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
香草醇氧化酶/对甲酚甲基羟化酶(VAO/PCMH)黄蛋白家族包括一系列能够催化各种底物氧化生物转化的酶。其中,来自4-烷基酚氧化亚基的松脂醇羟化酶(PinH)启动(+)-松脂醇的氧化降解,这是一种对木质素结构和植物防御都很重要的木脂素。在这项研究中,我们提出了详细的生化和结构表征假单胞菌sp. PinH,重点是其底物特异性和产物的形成。PinH在大肠杆菌中表达,纯化为含fad的可溶性蛋白。黄酶催化(+)-松脂醇和丁香酚的羟基化。结构分析显示其二聚体形式,非共价黄素结合,和一个大的活性位点。PinH-细胞色素复合物的AlphaFold模型证明了细胞色素在电子传递和调节PinH构象中的双重作用。PinH的一个显著特征是其多环(+)-树脂基板的大腔体。转化大块木脂素的能力对于旨在从酚类前体生产高价值化合物的生物技术应用特别有吸引力。这些见解扩大了我们对VAO/PCMH黄酶家族成员的结构和机制的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
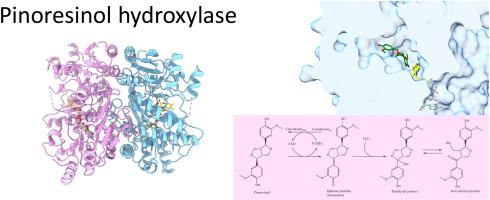
Biochemical and structural insights into pinoresinol hydroxylase from Pseudomonas sp
The vanillyl alcohol oxidase/p-cresol methylhydroxylase (VAO/PCMH) flavoprotein family comprises a broad spectrum of enzymes capable of catalyzing the oxidative bioconversions of various substrates. Among them, pinoresinol hydroxylase (PinH) from the 4-alkylphenol oxidizing subgroup initiates the oxidative degradation of (+)-pinoresinol, a lignan important for both lignin structure and plant defense. In this study, we present a detailed biochemical and structural characterization of PinH from Pseudomonas sp., with focus on its substrate specificity and product formation. PinH was expressed in E. coli and purified as FAD-containing, soluble protein. The flavoenzyme catalyzes the hydroxylation of both (+)-pinoresinol and eugenol. Structural analysis reveals its dimeric form, non-covalent flavin binding, and a large active site. AlphaFold models of the PinH-cytochrome complex demonstrate cytochrome's dual role in electron transfer and modulating PinH's conformation. A distinctive feature of PinH is a large cavity that hosts its multi-ring (+)-pinoresinol substrate. The capability of converting bulky lignans is particularly attractive for biotechnological applications aimed at producing high-value compounds from phenolic precursors. These insights expand our knowledge on the structure and mechanism of the VAO/PCMH flavoenzyme family members.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


